A kind of formula granule of Tiaowei Chengqi Decoction and its preparation method and detection method
A technology for Tiaowei Chengqi Decoction and formula granules, which can be used in pharmaceutical formulations, preparation of test samples, medical preparations containing active ingredients, etc., and can solve problems such as poor quality control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Preparation of Example 1 Tiaowei Chengqi Decoction Formula Granules
[0093] 1. Source of medicinal materials
[0094] See Table 1 for sources and places of origin.
[0095] Table 1
[0096]
[0097] 2. Instruments and equipment
[0098] HWZ-5B-1 box-type microwave vacuum drying equipment, Tianshui Huayuan Pharmaceutical Equipment Technology Co., Ltd.; RE-5205 rotary evaporator, Shanghai Yarong Biochemical Instrument Factory.
[0099] 3. Preparation method
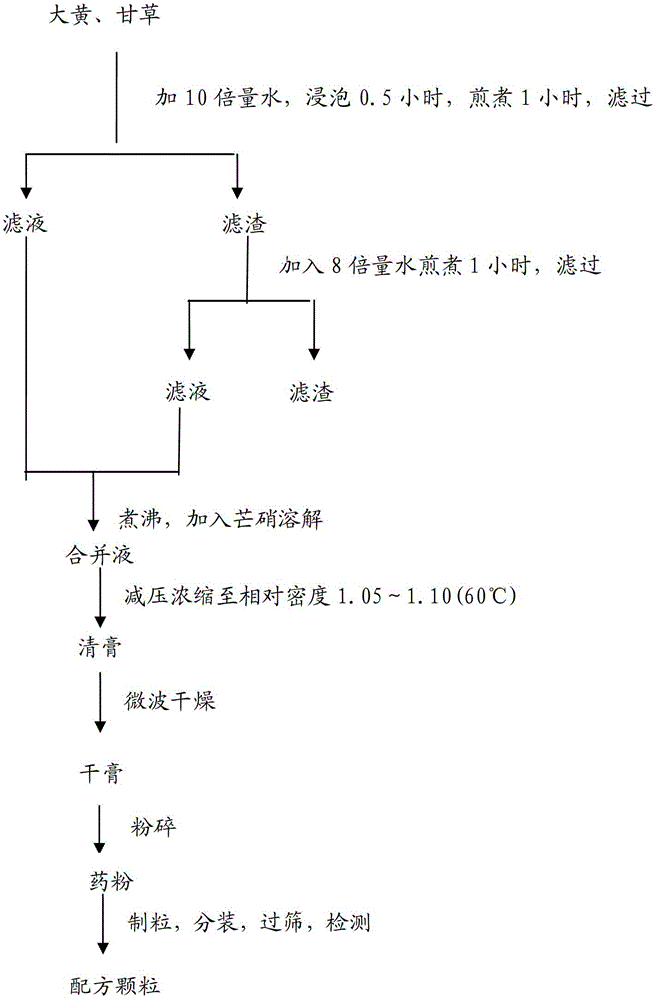
[0100] Take rhubarb and licorice and add water to decoct twice, filter, combine the filtrate, add Glauber’s salt to dissolve the filtrate after boiling, concentrate under reduced pressure at 70°C, and concentrate to a clear paste with a relative density of 1.05-1.10 (60°C), microwave drying, microwave power 5Kw, material temperature 50°C, running time 20min, drying to obtain dry paste granules, process flow chart see figure 1 .
[0101] The specific conditions are shown in Table 2 below:
[0102] Table 2...
Embodiment 2
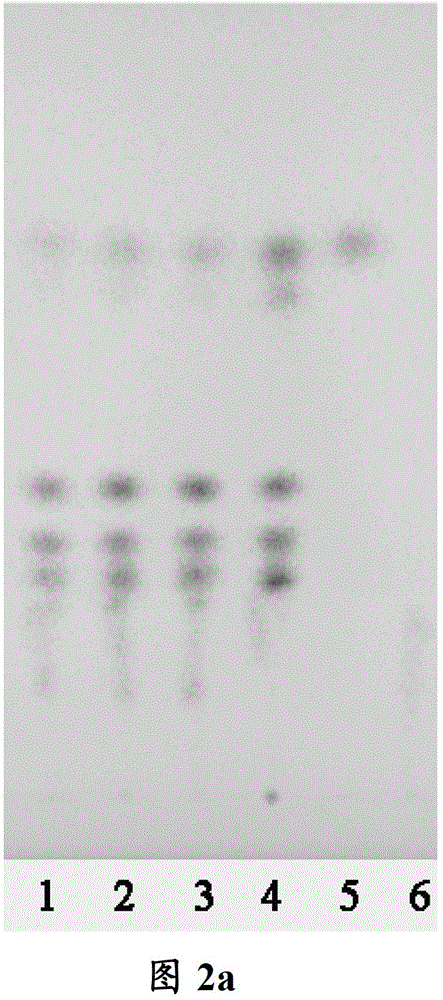
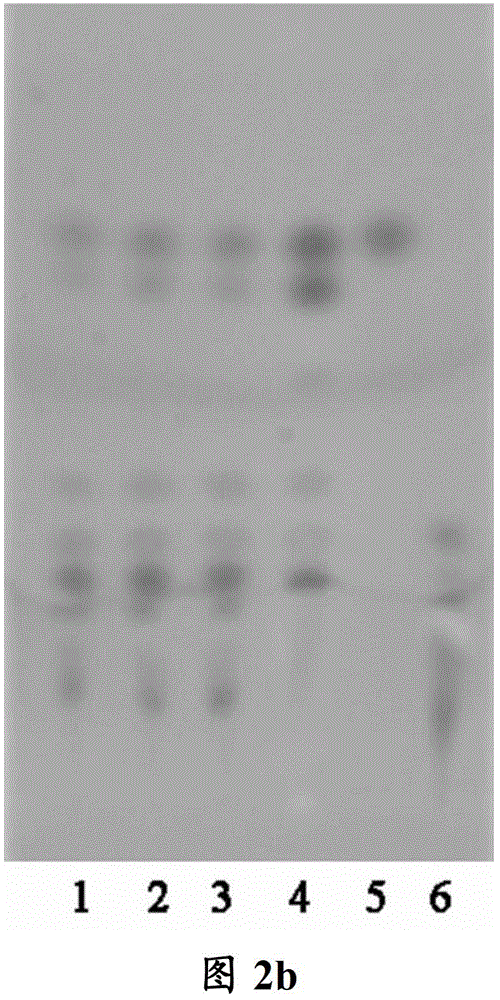
[0118] Embodiment 2 Thin-layer chromatography identification embodiment 1 is A in process condition 2 B 2 C 2 time-made tune Weichengqi Decoction Formula Granules
[0119] 1. Identification of Rhubarb in Tiaowei Chengqi Decoction Formula Granules
[0120] 1.1 Medicinal materials and reagents
[0121] Rhubarb control medicinal material: batch number 120984-200301, purchased from China Institute for the Control of Pharmaceutical and Biological Products.
[0122] Chrysophanol reference substance: batch number 110796-201017, purchased from China Institute for the Control of Pharmaceutical and Biological Products.
[0123] Test product: 12011001, produced by Kangmei Pharmaceutical Co., Ltd. according to Example 1 under process conditions A 2 B 2 C 2 time method.
[0124] TLC plate: Silicone G plate, made by Kangmei Pharmaceutical Co., Ltd.
[0125] 1.2 Test steps
[0126] Preparation of Rhubarb test solution: Take 2.5g of Tiaowei Chengqi Decoction formula gr...
Embodiment 3
[0153] Embodiment 3 detects that embodiment 1 is A at process condition 2 B 2 C 2 Tiao Wei Cheng Qi Decoction Heavy metal content of square particles
[0154] The test items refer to the Chinese Pharmacopoeia 2010 edition, under the [check] item of Huanglian Shangqing Pills, take about 1g of Tiaowei Chengqi Decoction formula granules prepared according to the method in Example 1 (batch number 12011003, made by Kangmei Pharmaceutical Co., Ltd.) , accurately weighed, according to the residue on ignition inspection method (Appendix IXJ of the Chinese Pharmacopoeia 2010 Edition) to be completely ashed. Take the remaining residue and check according to the law (Appendix IX E Second Method of Chinese Pharmacopoeia 2010 Edition), the results show that the heavy metal content does not exceed 25ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com