Olanzapine gastric soluble tablets and preparation method thereof
A stomach-dissolving, olanzapine technology, which is applied in the directions of pill delivery, pharmaceutical formulations, and inactive medical preparations, can solve the problems of unstable quality of finished products, numerous production steps, and many material losses, and achieves low cost. , The effect of short production cycle and stable quality of finished products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
[0033] Preparation: The dosage form can be produced by conventional tablet pharmaceutical equipment, and prepared by direct tableting process. The specific preparation method is as follows: grind the main drug olanzapine and silicon dioxide separately, pass through a 100-mesh sieve, weigh the prescription amount, and The two are mixed evenly. Microcrystalline cellulose and mannitol were respectively passed through a 60-mesh sieve, weighed according to the amount, and added to the above-mentioned mixed medicine in sequence and mixed evenly, then added the prescribed amount of magnesium stearate, sieved and mixed evenly, and carried out intermediate Body content detection, after determining the weight of the tablet, use the direct compression technology to compress the tablet, that is to say. The obtained tablet core is coated with Opadry Y-1-7000 alcohol solution, and the weight gain of the coating is 2% to 4%.
Embodiment 2
[0035] Obtain the tablet that is particularly suitable for each tablet that contains 1,2.5,5,7.5,10mg olanzapine respectively with following component weight and the method that embodiment 1 is substantially identical:
[0036] 1 mg olanzapine per tablet
[0037] ingredient name
[0038] 2.5 mg olanzapine per tablet
[0039] ingredient name
[0040] 5 mg olanzapine per tablet
[0041] ingredient name
[0042] Olanzapine
[0043] 7.5mg olanzapine per tablet
[0044] ingredient name
[0045] 10 mg olanzapine per tablet
[0046] ingredient name
[0047] Get the olanzapine tablet finished product of every 5mg specification in embodiment 2, and the dissolution rate in 0.1N HCL solution is 97% in 10min.
experiment example 1
[0048] Experimental example 1 Influencing factors investigation
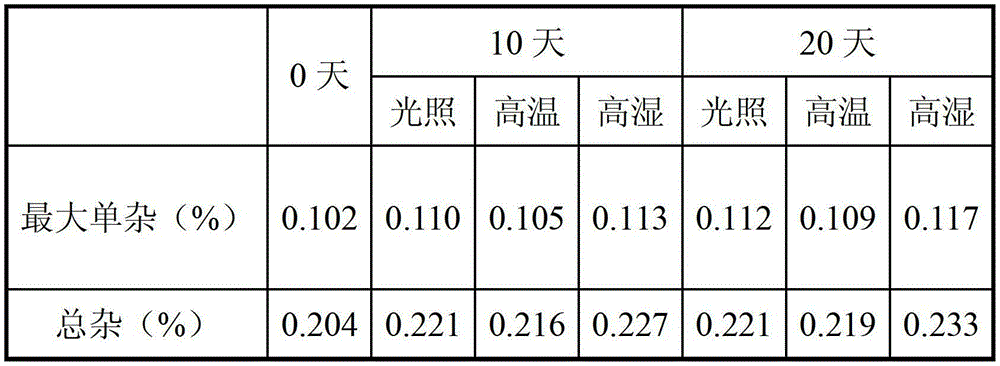
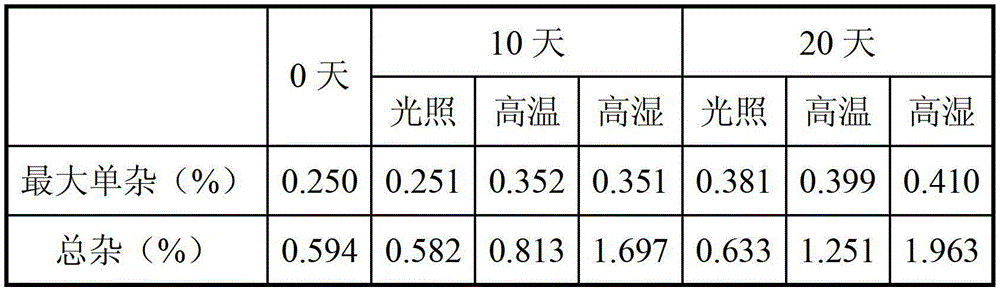
[0049] The finished olanzapine tablets of each 5mg specification in Example 2 were placed in a watch glass, and investigated under high temperature (60°C) and high humidity (RH92.5%) conditions. Specifically, the samples were placed in 60 ℃ incubator, RH92.5% environment and 4500LX light box, the final experimental results are as follows:
[0050]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com