Lyophilized composition for injection containing thymalfasin and preparation method thereof
A thymosin and composition technology, which is applied in the field of medicine, can solve the problems that the stability cannot meet the requirements of the stability of injections, potential safety hazards, and shorten the storage period of injections, and achieves high-quality reliability, less energy consumption, and residual moisture. less effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
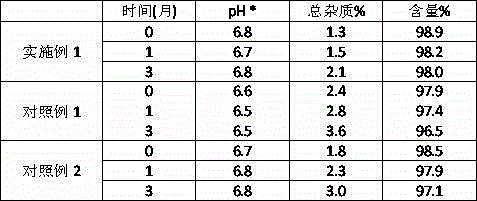
Embodiment 1
[0048] Weigh 50g of mannitol, disodium hydrogen phosphate (as Na 2 HPO 4 Calculated) 0.71g, sodium dihydrogen phosphate (as NaH 2 PO 4 Add 0.6g into the auxiliary material tank, add 1200mL water for injection, and stir to dissolve. Add the wetted activated carbon, stir and absorb for 20 minutes, then turn off the stirring, use clean compressed air as the power, pass the solution through a titanium rod, decarbonize, and put it into the liquid mixing tank, and rinse the auxiliary materials with water for injection to obtain the first filtrate. Add water for injection to 1800mL in the batching tank, control the water temperature at 25 o Below C, stir, and after detecting that the pH of the solution is 6.4~7.2, slowly add 1.6g of thymofasin into the batching tank, and stir to dissolve. After detecting that the pH of the solution is 6.4~7.2, continue to add water for injection to 2000mL, and after stirring for 5~15 minutes, filter the medicinal solution through a 0.22μm micropo...
Embodiment 2
[0051] Weigh 50g of mannitol, disodium hydrogen phosphate (as Na 2 HPO 4 Calculated) 0.71g, sodium dihydrogen phosphate (as NaH 2 PO 4 Add 0.6g into the auxiliary material tank, add 1000mL water for injection, and stir to dissolve. Add the wetted activated carbon, stir and absorb for 20 minutes, then turn off the stirring, use clean compressed air as the power, pass the solution through a titanium rod, decarbonize, put it into the liquid mixing tank, and rinse the auxiliary materials with water for injection to obtain the first filtrate. Add water for injection to 1300mL in the batching tank, control the water temperature at 25 o Below C, stir, slowly add 1.6g of thymus fasin into the batching tank, stir to dissolve. Continue to add water for injection to 1500mL, continue to stir for 5-15 minutes, then filter the medicinal solution through a 0.22μm microporous membrane to obtain the second filtrate, and divide the filtrate into vials, each 1.5mL.
[0052] Freeze-drying pr...
Embodiment 3
[0054] Weigh 50g of mannitol, disodium hydrogen phosphate (as Na 2 HPO 4 Calculated) 0.71g, sodium dihydrogen phosphate (as NaH 2 PO 4 Add 0.6g into the auxiliary material tank, add 2200mL water for injection, and stir to dissolve. Add the wetted activated carbon, stir and absorb for 20 minutes, then turn off the stirring, use clean compressed air as the power, pass the solution through a titanium rod, decarbonize, put it into the liquid mixing tank, and rinse the auxiliary materials with water for injection to obtain the first filtrate. Control the water temperature at 25 o Below C, stir, slowly add 1.6g thymofasin into the batching tank, stir to dissolve. Continue to add water for injection to 2500mL, continue to stir for 5-15 minutes, then filter the medicinal solution through a 0.22μm microporous membrane, and divide the filtrate into 2.5mL vials.
[0055] Freeze-drying process: same as Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com