Photoreactive cell membrane structure-imitated copolymer and preparation method and application thereof
A technology of structural polymer, imitating cell outer layer membrane, applied in the direction of coating, etc., can solve the problems of difficult to achieve control results, the interaction of coating polymer segments and group migration orientation and condensation and cross-linking process, etc. The effect of good hydrophilic performance and biocompatibility, stable coating structure and simple treatment process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] 1. Preparation of amino-containing copolymer PMBA
[0034] Using the "starvation method" (Lewis AL, et al . Biomaterials 2000, 21: 1847-1859) Synthesis of (meth)acryloyloxyethyl phosphorylcholine, (meth)acrylic acid ester and (meth)acrylic acid-2-aminoethyl ester terpolymer random copolymer (referred to as PMBA) . Monomer (meth)acryloyloxyethylphosphorylcholine, (meth)acrylate and 2-aminoethyl (meth)acrylate are mixed and dissolved in isopropanol or ethanol. Weigh 0.5~1.5% initiator AIBN of the total mass of the monomer and dissolve it in THF. Add a THF solution of initiator AIBN with a mass fraction of 15-40% into the reactor, and then add the monomer and the remaining initiator mixture at a speed of 30-80% per hour. The molar percentages of the hydrophilic monomer containing phosphorylcholine group, the hydrophobic monomer containing alkyl chain and the monomer containing amino group are 25-80%, 0-50% and 5-50%, respectively. The content of each unit in the PM...
Embodiment 1
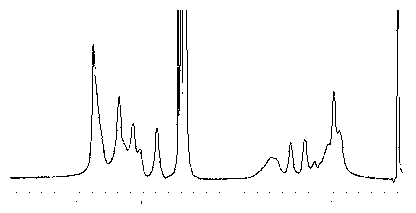
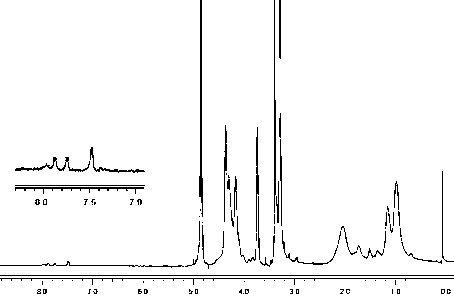
[0040] Take methacryloyloxyethylphosphorylcholine (1.20 g), n-butyl methacrylate (0.65 g) and 2-aminoethyl methacrylate (0.45 g), dissolve them in isopropanol to synthesize ternary Random copolymer Poly(MPC- co -MB- co -MAE) abbreviated as PMBA442. Weigh the initiator AIBN of 1.0% of the total mass of the monomer, add 1 / 4 volume to the reactor after dissolving with THF, mix 3 / 4 with the monomer solution, and 2 Under protection, the mixture of monomer and initiator was added dropwise in a constant temperature and stirred reactor at 80°C to carry out starvation copolymerization of monomer, and the drop was completed within 2 hours. The reaction was stopped after 24 h of reaction after the dropwise addition. Precipitate and separate with ether to prepare 5% ethanol solution, purify by dialysis with a molecular weight cut-off of 8000 dialysis bag, and obtain 1.13 g of PMBA442 copolymer after freeze-drying. NMR results: 1H NMR (δ / ppm): 3.7 (CH 2 N + ), 1.2-1.5((CH 2 ) 2 )...
Embodiment 2
[0043] Take methacryloyloxyethylphosphorylcholine (2.08 g), n-butyl methacrylate (0.48 g) and 2-aminoethyl methacrylate (1.13 g), dissolve them in isopropanol to synthesize a ternary Random copolymer Poly(MPC- co -MB- co -MAE) abbreviated as PMBA424. Weigh the initiator AIBN of 1.2% of the total mass of the monomer, add 1 / 4 volume to the reactor after dissolving with THF, mix 3 / 4 with the monomer solution, and put it under N 2 Under protection, the mixture of monomer and initiator was added dropwise in a constant temperature and stirred reactor at 80°C to carry out monomer starvation copolymerization reaction, and the drop was completed within 3 h. After 24 h of reaction, the reaction was stopped. Precipitation and separation with ether was used to prepare a 5% ethanol solution, which was purified by dialysis with a molecular weight cut-off of 8000 and lyophilized to obtain 1.50 g of PMBA424 copolymer. NMR results: 1H NMR (δ / ppm): 3.7 (CH 2 N + ), 1.2-1.5((CH 2 ) 2 )...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com