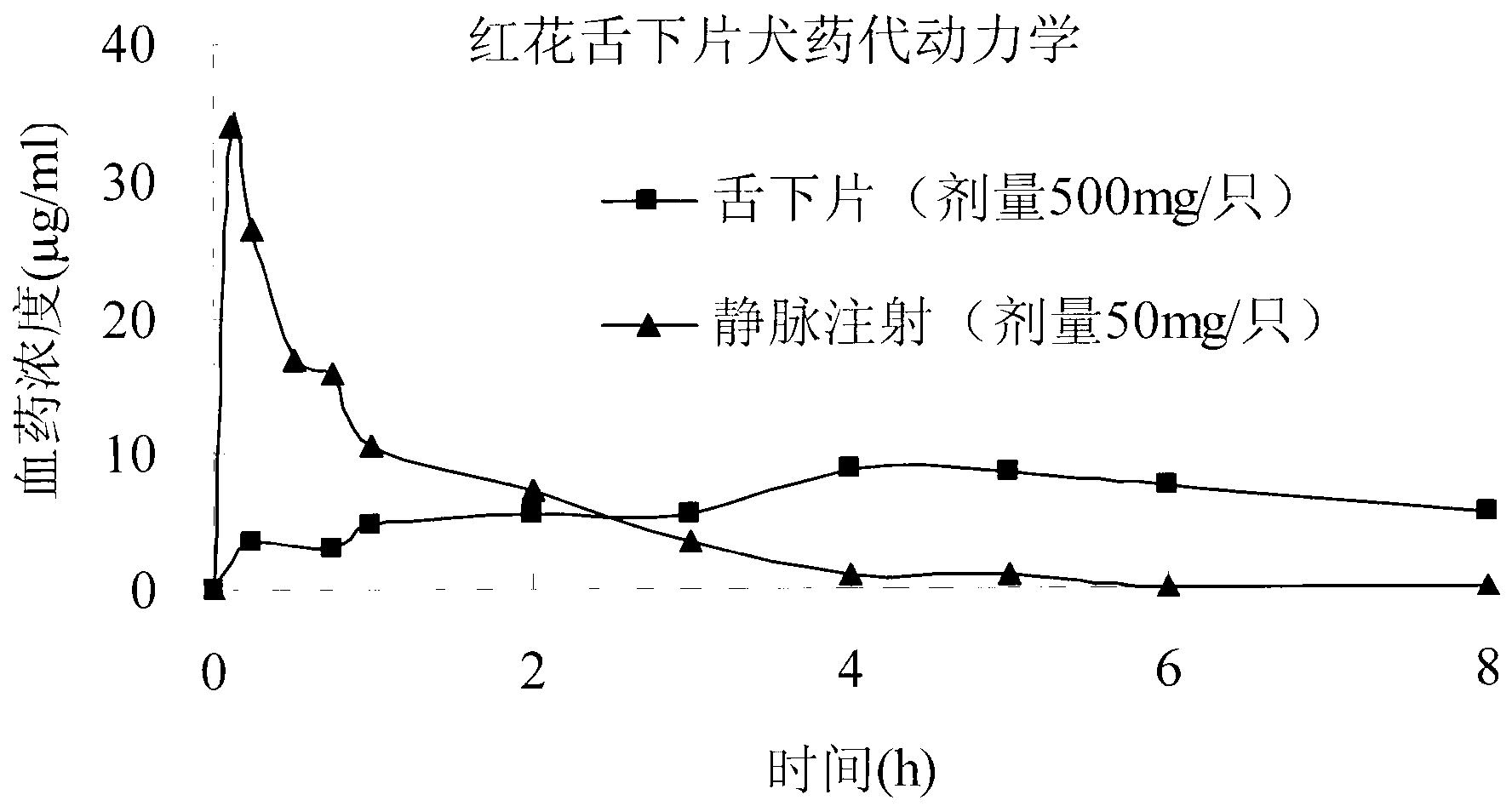
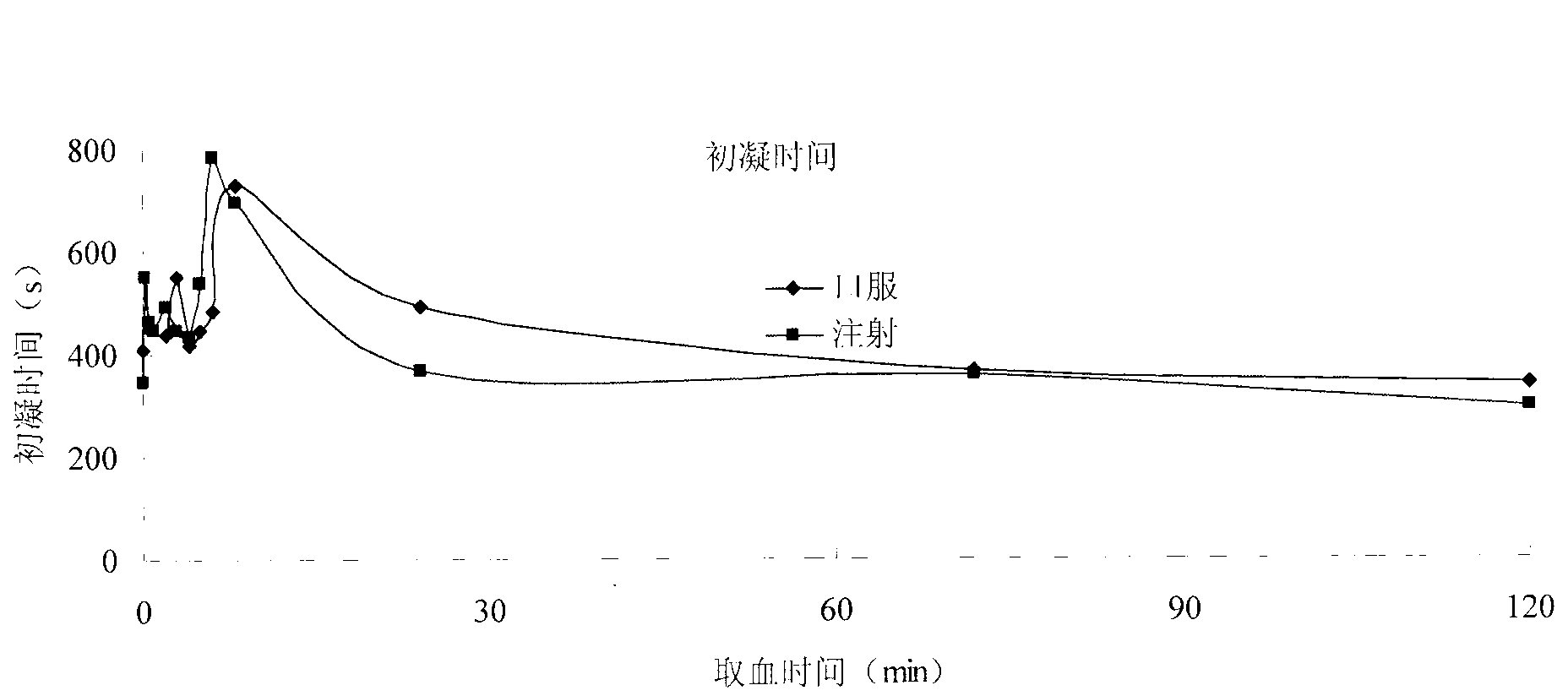
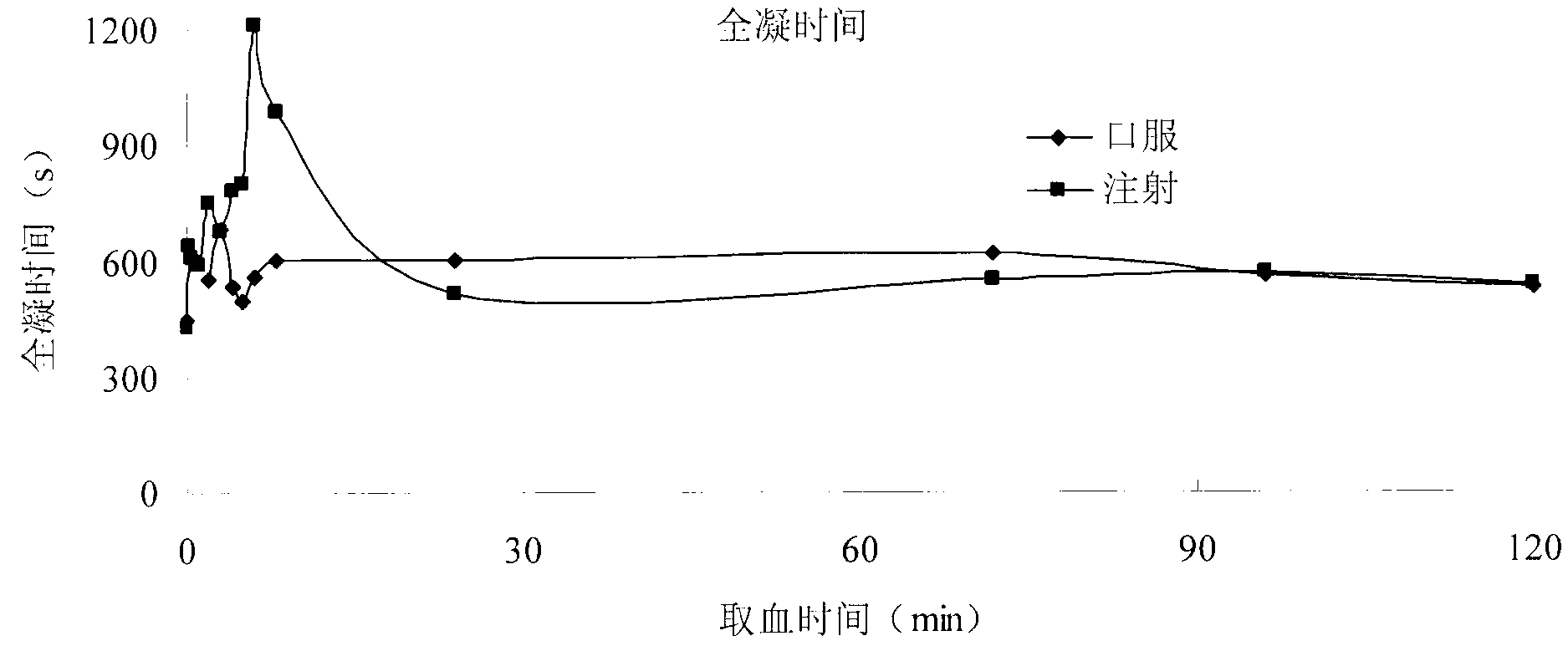
Carthamin yellow sublingual tablet and preparation method and application thereof
A technology of safflower yellow pigment and sublingual tablet, which can be applied to pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc. Large and other problems, to achieve the best disintegration effect, good taste and appearance, and the effect of rapid absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 different fillers
[0026] Using different types of fillers as shown in Table 1, according to the formula of 100mg per tablet containing safflower yellow pigment, 400mg filler, 100mg croscarmellose sodium and 5mg magnesium stearate, it is prepared by powder direct compression method slices, and the results are shown in Table 1.
[0027] Table 1
[0028] serial number
[0029] As can be seen from the results in Table 1, mannitol, sorbitol, xylitol and lactose can all be formed into tablets and have better mouthfeel, especially mannitol, sorbitol and xylitol can realize better mouthfeel. In addition, considering the high price of sorbitol and xylitol, it is most preferable to use mannitol.
Embodiment 2
[0030] The safflower yellow pigment sublingual tablet of embodiment 2 different disintegrants and preparation method
[0031] 1. Preparation of powder by direct compression method
[0032] Using different types of disintegrants as shown in Table 2, according to the formula containing 100mg safflower yellow pigment, 300mg lactose, 200mg disintegrant and 5mg magnesium stearate per tablet, prepared by powder direct compression method, the results are shown in the table 2.
[0033] Table 2
[0034] serial number
[0035] 2. Wet granulation preparation
[0036] Adopt different kinds of disintegrants as shown in table 3, contain 100mg safflower yellow pigment, 400mg mannitol, 100mg disintegrator and the prescription of 5mg magnesium stearate by every tablet, with following wet granulation tabletting method (additional method), and the results are shown in Table 3.
[0037] Wet granulation (external addition method): weigh safflower yellow and mannitol according to the...
Embodiment 3
[0041] Example 3 Safflower Yellow Sublingual Tablets with Different Proportions
[0042] Formula (mg / tablet): Except for fillers and disintegrants as shown in Table 4, others are: 100 mg of safflower yellow, 5 mg of magnesium stearate.
[0043] Preparation method: powder direct compression method
[0044] Table 4
[0045] serial number
Disintegration time (n=6)
Disintegration time limit
1
500mg
0mg
9-10min
unqualified
2
450mg
50mg
6-7min
unqualified
3
400mg
100mg
4-5min
qualified
4
300mg
200mg
4min
qualified
[0046] It can be seen from the results in Table 4 that when the dosage ratio of mannitol and croscarmellose sodium is 4:1, the qualified disintegration time limit has been reached, and it is not meaningful to increase the dosage of disintegrating agent, and it will make the taste worse, so Most preferred i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com