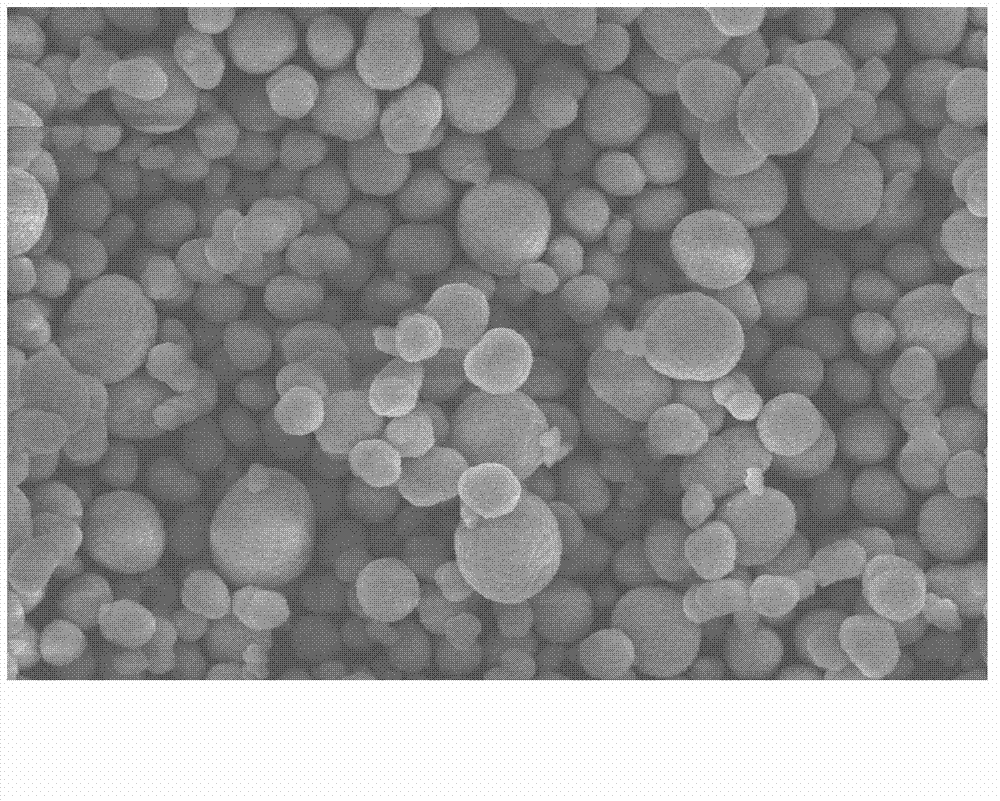
Method for preparing nickel-cobalt lithium aluminate as anode material of lithium ion battery
A nickel-cobalt-aluminate lithium-ion battery technology, applied in battery electrodes, circuits, electrical components, etc., can solve problems such as difficult co-precipitation of aluminum ions, and achieve the effect of improving the electrical properties of materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] A method for preparing lithium-ion battery cathode material nickel-cobalt-aluminate lithium, comprising the following steps:
[0022] (1) Use nickel sulfate and cobalt sulfate to prepare 200 liters of a solution with a nickel-cobalt molar ratio of 8:1.5 and a total nickel-cobalt molar concentration of 2.0 mol / L;
[0023] (2) Prepare 100 liters of 0.2 mol / L sodium metaaluminate solution with 4.0 mol / L sodium hydroxide solution;
[0024] (3) Put the above-mentioned prepared metal salt solution and sodium metaaluminate solution into the reaction kettle through a certain flow rate, and at the same time add sodium hydroxide as a precipitant and ammonia as a complexing agent, and control the reaction temperature in the reaction kettle to 55 ℃, the concentration of ammonia water is 10.0g / L, and the reaction pH value is 11.50. After 40 hours of reaction, filter the precipitate, wash the precipitate with pure hot water at 80°C, and put the precipitate in a 110°C drying oven to d...
Embodiment 2
[0034] A method for preparing lithium-ion battery cathode material nickel-cobalt-aluminate lithium, comprising the following steps:
[0035] (1) Use nickel sulfate and cobalt sulfate to prepare 200 liters of a solution with a nickel-cobalt molar ratio of 7:2.5 and a total molar concentration of nickel-cobalt-manganese of 2.0 mol / L;
[0036] (2) Prepare 100 liters of 0.2 mol / L sodium metaaluminate solution with 4.0 mol / L sodium hydroxide solution;
[0037] (3) Put the above-mentioned prepared metal salt solution and sodium metaaluminate solution into the reaction kettle through a certain flow rate, and at the same time add sodium hydroxide as a precipitant and ammonia as a complexing agent, and control the reaction temperature in the reaction kettle to 30 ℃, the concentration of ammonia water is 10.0g / L, and the reaction pH value is 9.0. After 200 hours of reaction, filter the precipitate, wash the precipitate with pure hot water at 50°C, and dry the precipitate in a 110°C dryi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com