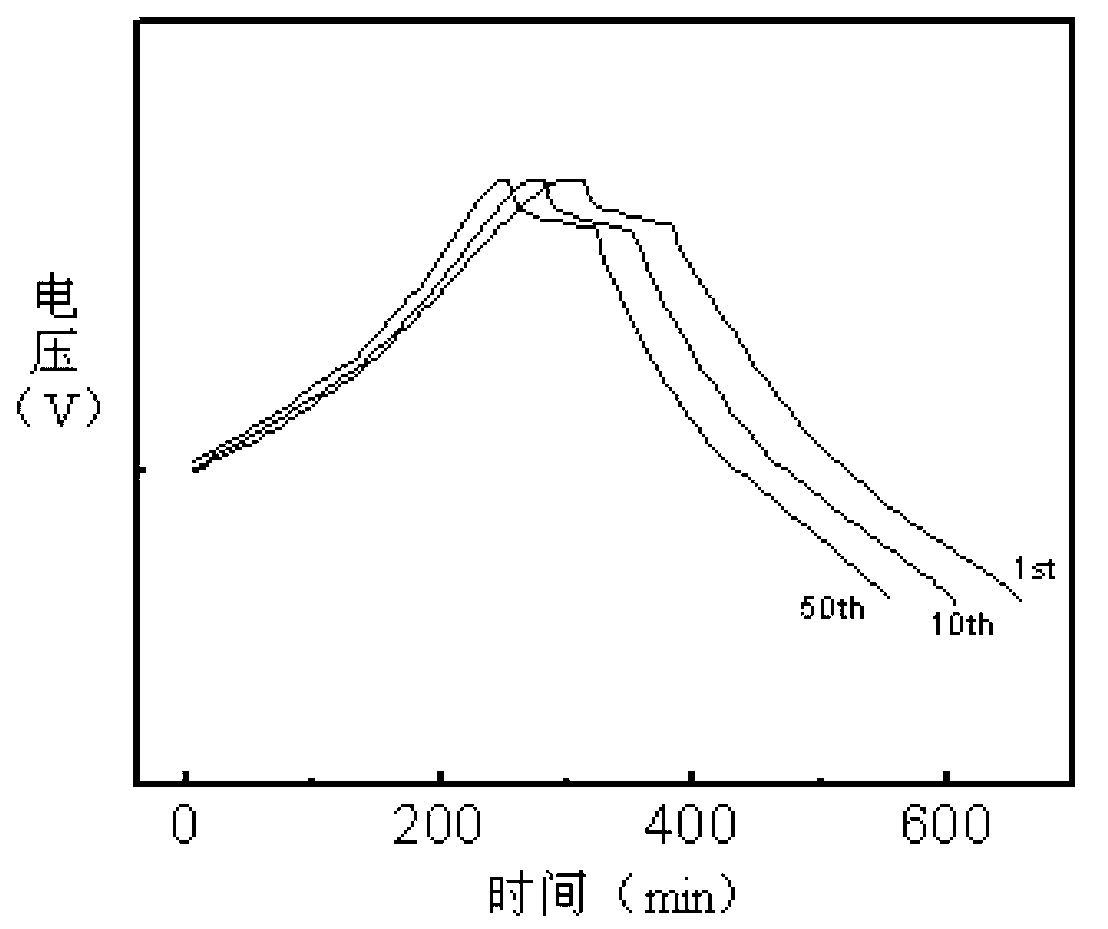
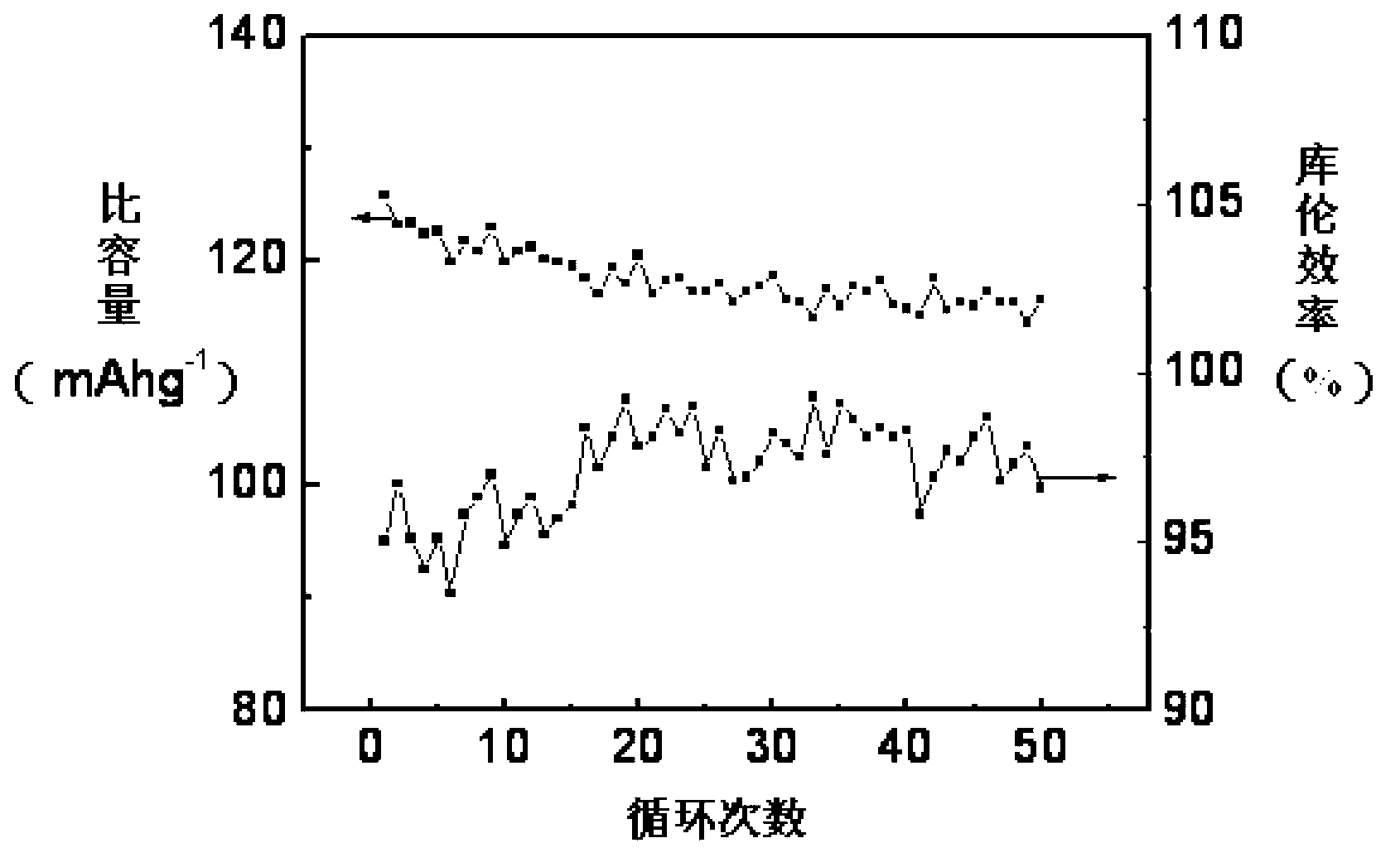
Synthetic method of electrode material polyaniline
A synthesis method and electrode material technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of low yield of polyaniline, achieve the effects of improving utilization, improving electrical activity, and increasing yield and capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The invention provides a kind of synthetic method of electrode material polyaniline, comprises the following steps:
[0034] a) dissolving aniline in perchloric acid solution, reacting in the presence of ferric sulfate or manganese dioxide at a temperature below 10°C to obtain a reaction solution;
[0035] b) adding the perchloric acid solution dissolved in ammonium persulfate dropwise in batches to the reaction liquid obtained in step a), and continuing the reaction at a temperature below 10° C. to obtain polyaniline as an electrode material.
[0036]In the embodiment of the present invention, aniline, iron sulfate or manganese dioxide are added to the perchloric acid solution for reaction, and the temperature is controlled not to exceed 10° C. to obtain a reaction solution.
[0037] The present invention uses perchloric acid as an acidic reaction medium, and in this strongly acidic reaction system, aniline can exist in the form of aniline salt (aniline acidified ion),...
Embodiment 1
[0058] Add 46mL perchloric acid to 250mL deionized water, stir 3min, prepare perchloric acid solution, then take out 50mL described perchloric acid solution for subsequent use, and add 1.2g manganese dioxide in the described perchloric acid solution of surplus, Cool down to 5°C to obtain a perchloric acid solution containing manganese dioxide;
[0059] Add 5 mL of perchloric acid to 95 mL of deionized water, then add 26.5 g of ammonium persulfate, stir to dissolve, then cool down to 5°C to obtain a perchloric acid solution in which ammonium persulfate is dissolved;
[0060] Add 1.6g of phenolic carbon to the spare 50mL perchloric acid solution, stir for 15 minutes, ultrasonically disperse for 10 minutes, then add 9.6g of aniline, and stir for 10 minutes to dissolve the aniline in the perchloric acid solution to obtain aniline phenolic carbon mixed liquid;
[0061]Pour the aniline-phenol-formaldehyde-carbon mixed solution into the cooled perchloric acid solution containing man...
Embodiment 2
[0068] Add 1.2L of perchloric acid to 6L of deionized water, stir for 3 minutes, add 18.9g of manganese dioxide, and cool down to 5°C (take 300mL of water and put it in the refrigerator in advance to make ice cubes to increase the cooling speed), and the obtained manganese perchloric acid solution;
[0069] Add 150mL of perchloric acid to 2.8L of deionized water, then add 1200g of ammonium persulfate, stir to dissolve and cool down to 5°C to obtain a perchloric acid solution in which ammonium persulfate is dissolved;
[0070] Add 180mL of perchloric acid to 1.2L of deionized water, lower the temperature to below 10°C to obtain a perchloric acid solution, add 420g of aniline to it, then add 126g of phenolic carbon, stir for 1 hour, and dissolve the aniline in the perchloric acid solution , to obtain aniline phenolic carbon mixture;
[0071] Pour the aniline-phenol-formaldehyde-carbon mixture into the cooled perchloric acid solution containing manganese dioxide for reaction, fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com