N-substituted gardenamide A derivative and synthesis method and application thereof
A synthesis method and technology of derivatives, applied in the preparation of drugs for the treatment of senile dementia, N-substituted gardenamide A derivatives and its synthesis field, can solve problems such as poor stability, achieve mild reaction conditions, high yield, The effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation, separation and purification of N-methyl gardenamide A (i.e. compound 1) includes the following steps:
[0048] (1) Preparation of 7-(O-dimethyltert-butylsilyloxy)methylgenipin: Add genipin 0.339g (1.5mmol) and 0.5mL dry DMF into a 10mL reaction flask, then add imidazole 0.204 g (3.0mmol), and finally a DMF solution containing 0.27g (1.8mmol) of dimethyl tert-butylsilyl chloride (TBS-Cl) was added dropwise, stirred at room temperature for 1h, then added 100mL DCM for extraction, washed 3 times with water (3 ×100ml), the organic phase was dried with anhydrous magnesium sulfate, filtered, and concentrated by rotary evaporation to obtain a light yellow oily solid, which was separated by column chromatography of a mixed solution of petroleum ether-ethyl acetate (volume ratio 9:1) to obtain a white solid 0.453 g, yield 89.0%.
[0049] The structural characterization data of the product are as follows, which is 7-(O-dimethyltert-butylsilyloxy)methylgenipin. ...
Embodiment 2
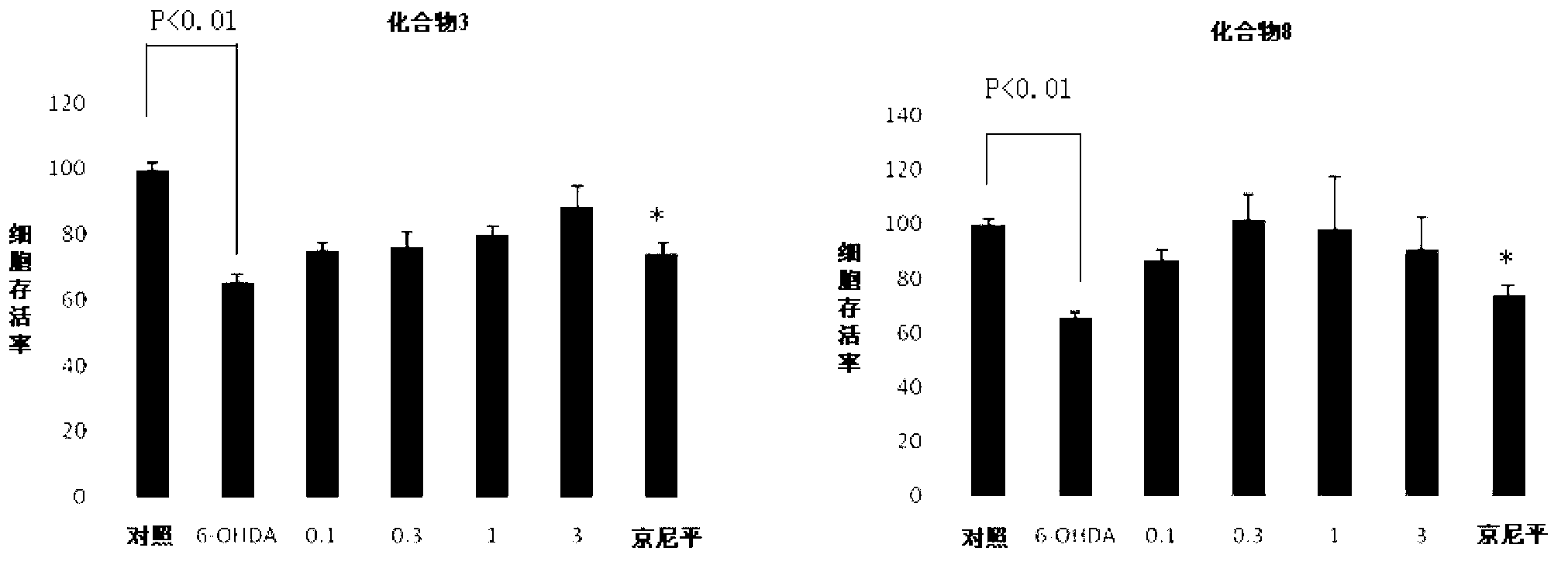
[0059] The preparation and separation and purification of N-isopropyl gardenamide A (i.e. compound 3), the steps and raw materials are the same as in Example 1, only in step 3, 0.1ml (1.2mmol) of isopropylamine was used instead of methylamine hydrochloride, and finally After separation and purification by HPLC, 20.7 mg of a white solid compound was obtained, with a yield of 52%.
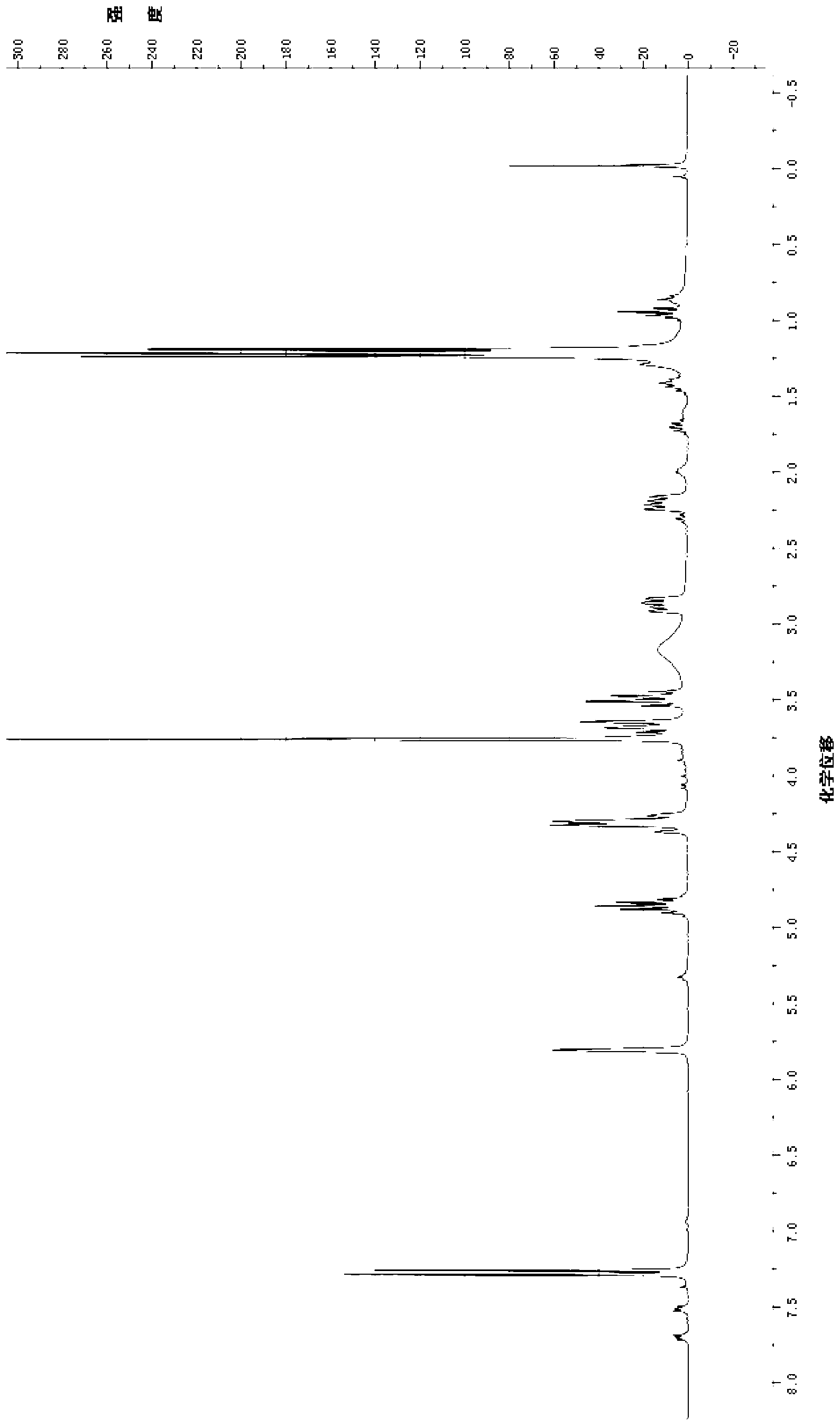
[0060] The structural characterization data of the product are: 1 H NMR (300MHz, CDCl 3 )δ:7.29(s,1H),5.81(s,1H),4.90-4.81(m,1H),4.37-4.27(m,2H),3.76(s,3H,-OCH 3 ),3.64(d,J=11.1Hz,1H),3.51-3.45(m,1H),2.91-2.83(m,1H),2.25-2.16(m,1H),1.21(t,J=6.9Hz, 6H); 13 C NMR (75MHz, CDCl 3 )δ: 169.96(C-11), 166.89(C-1), 141.30(C-3), 132.58(C-8), 129.27(C-7), 111.57(C-4), 60.99(C-10 ),51.60(-OCH 3 ),50.65(-NCH),45.25(C-9),40.00(C-6),36.82(C-5),21.06(-CH( C h 3 ) 2 ),20.54(-CH( C h 3 ) 2 );ESI-MS(m / z):288.2[M+Na] + . 1 H NMR spectrum see figure 1 , proving that the structure of the product is like com...
Embodiment 3
[0062]Preparation, separation and purification of N-phenyl gardenamide A (namely compound 6), the steps and raw materials are the same as in Example 1, only in step 3, 0.1ml (1.1mmol) of aniline was used instead of methylamine hydrochloride, and finally separated by HPLC After purification, 27.4 mg of white solid compound was obtained, with a yield of 61%.
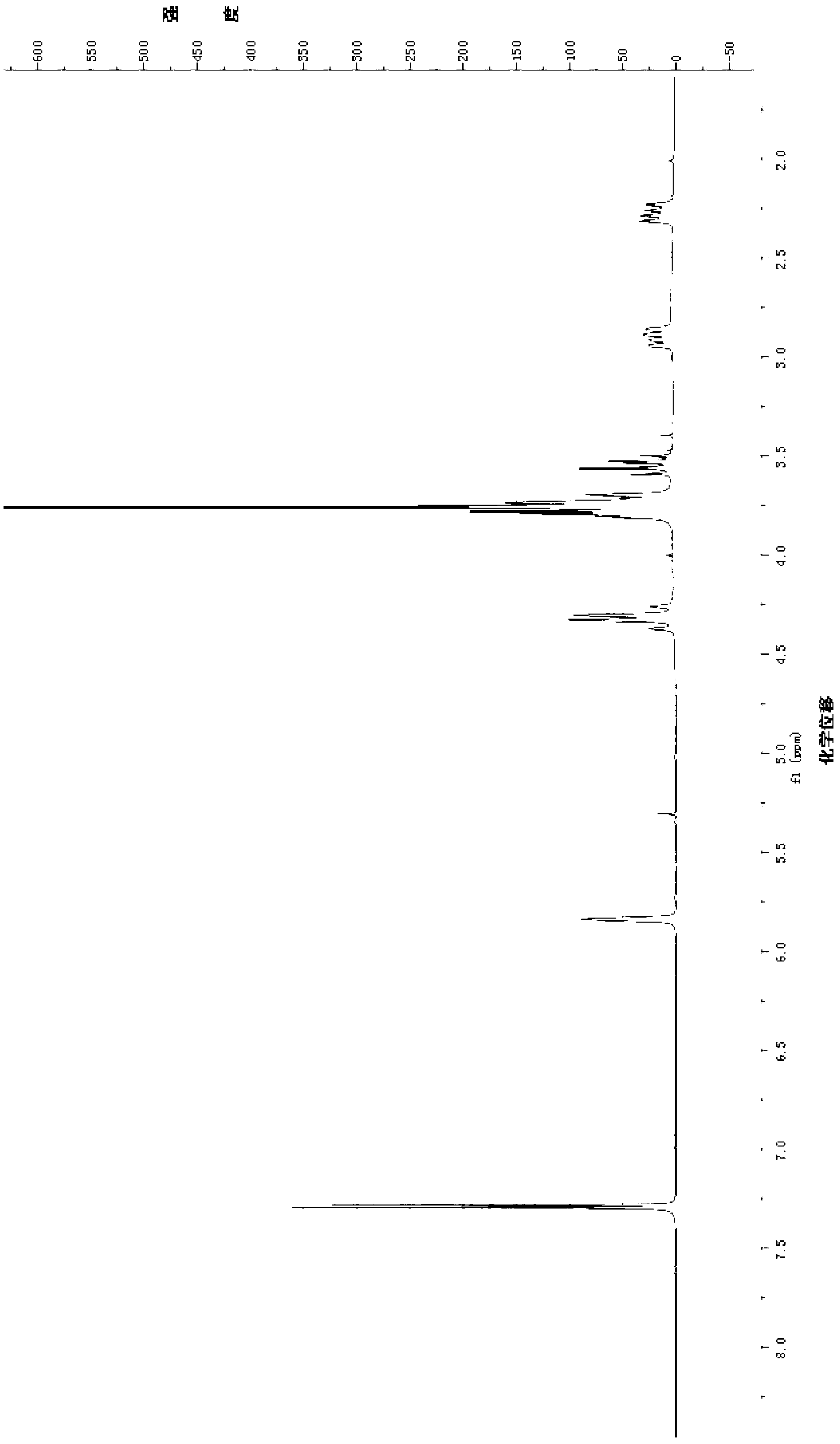
[0063] The structural characterization data of the product are: 1 H NMR (300MHz, CDCl 3 )δ:7.47-7.37(m,4H,Ar-H),7.27-7.24(m,1H,Ar-H),7.24(s,1H),5.88(s,1H),4.36(q,J=13.5 Hz,2H),3.89(d,J=11.1Hz,1H),3.76(s,3H,-OCH 3 ),3.70-3.61(m,1H),3.04-2.94(m,1H),2.43-2.33(m,1H); 13 C NMR (75MHz, CDCl 3 )δ: 170.27(C-11), 166.71(C-1), 141.21(C-3), 139.78(C 6 h 5 ), 137.91(C-8), 129.38(C 6 h 5 ), 129.14(C-7), 128.25(C 6 h 5 ), 126.36 (C 6 h 5 ), 111.34 (C-4), 60.88 (C-10), 51.64 (-OCH 3 ),50.45(C-9),40.11(C-6),37.34(C-5);ESI-MS(m / z):300.2[M+H] + . The structure of the product was proved to be compound 6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com