A lyophilized preparation of a cytochrome C-containing pharmaceutical composition for injection and a preparation method thereof
A technology of cytochrome and freeze-dried preparations, applied in the field of medicine, can solve problems such as unstable content of cytochrome C, unqualified foreign matter in clarity, unstable product quality, etc., shorten the freeze-drying time, increase hardware investment, and stabilize Guaranteed effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
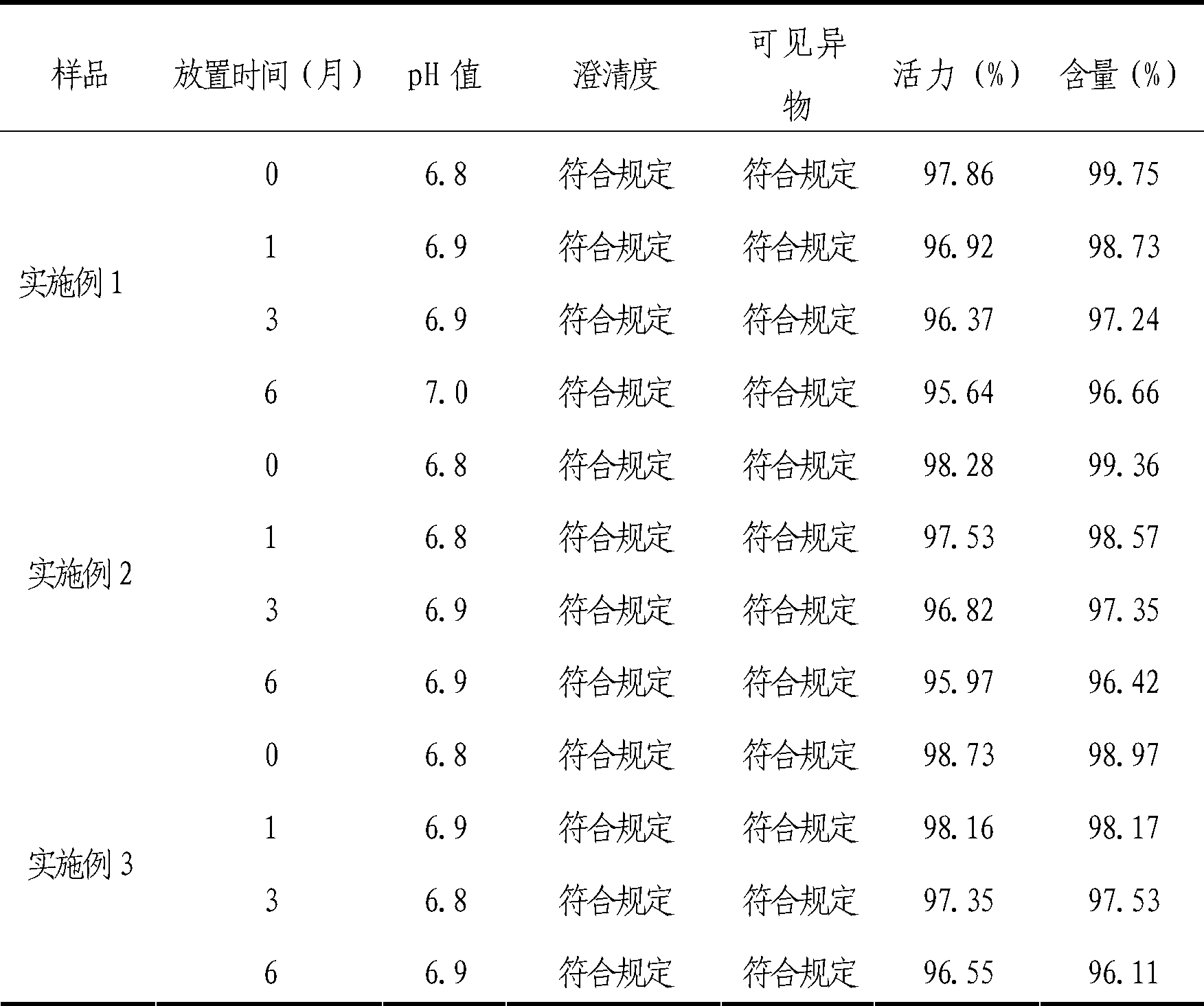
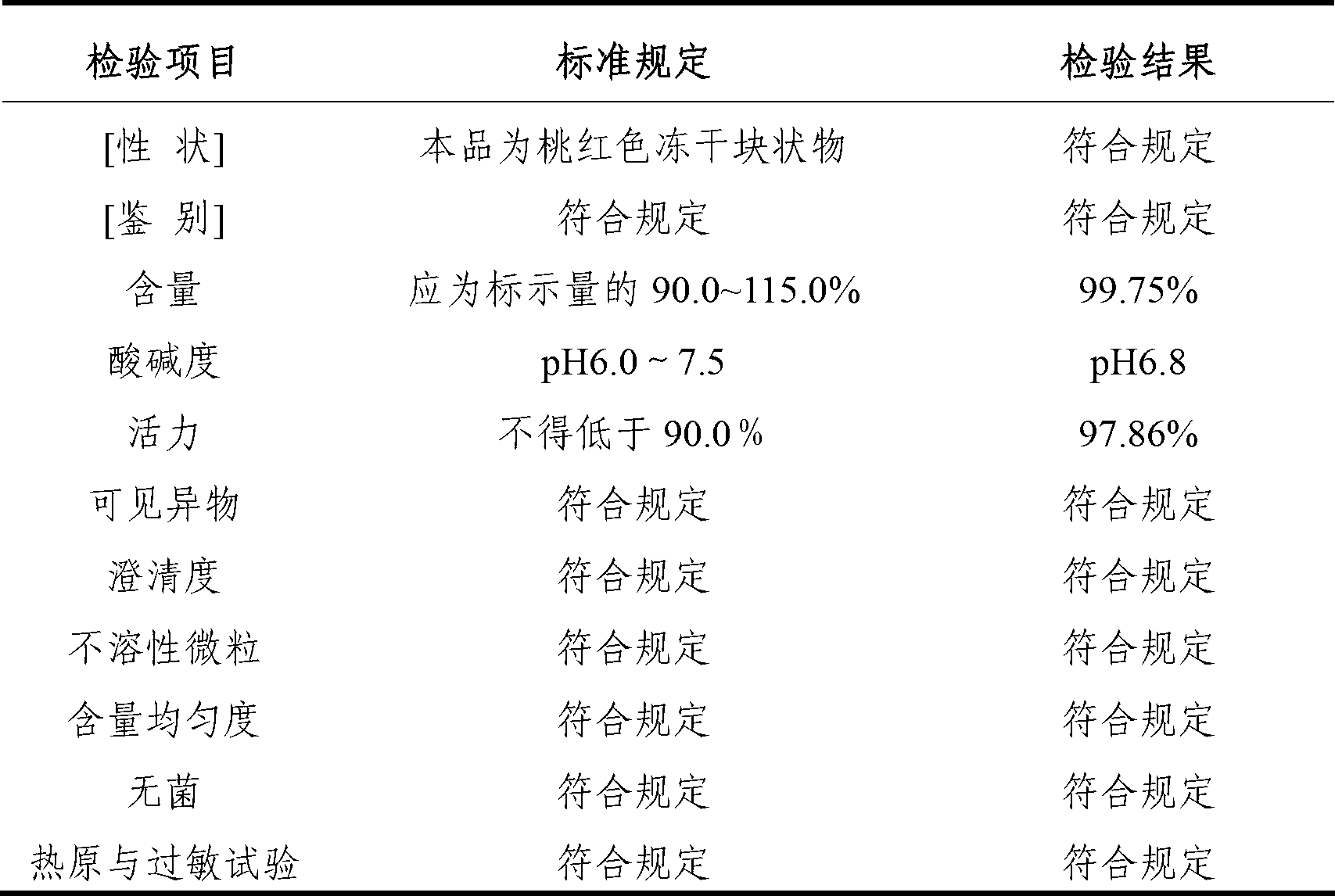
[0032] The freeze-dried preparation of the cytochrome C-containing pharmaceutical composition for injection of the present invention is made of the following components: cytochrome C 7.5g; L-arginine 3g; edetate disodium 0.20g; mannitol 15g, water for injection to 1000ml.
[0033] Its preparation method is as follows:
[0034] 1) Weigh the prescribed amount of mannitol, add 500ml of water for injection to dissolve, add 2‰ activated carbon, boil, keep warm for 15 minutes, filter and decarbonize to obtain solution ①.
[0035] 2) Cool the solution ① to below 20°C, add the prescribed amount of L-arginine and edetate disodium successively to obtain the solution ②.
[0036] 3) Take the prescribed amount of cytochrome C solution, add water for injection to make up to 300 ml, and obtain solution ③.
[0037] 4) Mix solution ② and solution ③, and then make up the volume to the total amount with cold water for injection, adjust the pH value to 6.7, filter through a 0.22μm pore size ste...
Embodiment 2
[0045] The lyophilized formulation of the cytochrome C-containing pharmaceutical composition for injection of the present invention is made of the following components: cytochrome C 15g; L-arginine 4.5g; edetate disodium 0.3g; mannitol 15g, water for injection added to 1000ml.
[0046] Its preparation method is as follows:
[0047] 1) Weigh the prescribed amount of mannitol, add 500ml of water for injection to dissolve, add 2‰ activated carbon, boil, keep warm for 15 minutes, filter and decarbonize to obtain solution ①.
[0048] 2) Cool the solution ① to below 20°C, add the prescribed amount of L-arginine and edetate disodium successively to obtain the solution ②.
[0049] 3) Take the prescribed amount of cytochrome C solution, add water for injection to make up to 300 ml, and obtain solution ③.
[0050] 4) Mix solution ② and solution ③, and then make up the volume to the total amount with cold water for injection, adjust the pH value to 6.8, filter through a 0.22μm pore siz...
Embodiment 3
[0058] The freeze-dried preparation of the cytochrome C-containing pharmaceutical composition for injection of the present invention is made of the following components: cytochrome C 30g; L-arginine 6g; edetate disodium 0.50g; mannitol 15g, water for injection is added to 1000ml .
[0059] Its preparation method is as follows:
[0060] 1) Weigh the prescribed amount of mannitol, add 500ml of water for injection to dissolve, add 2‰ activated carbon, boil, keep warm for 15 minutes, filter and decarbonize to obtain solution ①.
[0061] 2) Cool the solution ① to below 20°C, add the prescribed amount of L-arginine and edetate disodium successively to obtain the solution ②.
[0062] 3) Take the prescribed amount of cytochrome C solution, add water for injection to make up to 300 ml, and obtain solution ③.
[0063] 4) Mix solution ② and solution ③, and then make up the volume to the total amount with cold water for injection, adjust the pH value to 6.9, filter through a 0.22μm pore...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com