P-coumaroyl ester 3'-hydroxylase gene LjC3'H in lonicera japonica thumb. and application thereof
A technology of coumaryl ester and honeysuckle, applied in application, genetic engineering, plant gene improvement, etc., can solve the problems of unreported research on the relationship with chlorogenic acid, cloning, and functional verification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Cloning of embodiment 1, LjC3'H
[0027] 1.1 Extraction of honeysuckle total RNA
[0028] (1) Put the honeysuckle material into a pre-cooled mortar, add liquid nitrogen and quickly grind it into a uniform powder. Pay attention to ensure that the material is immersed in liquid nitrogen during the grinding process.
[0029] (2) After the liquid nitrogen volatilizes, quickly transfer the material to a pre-cooled centrifuge tube, add 1ml TRIZOL solution for every 50-100mg of tissue material, mix well, place it at room temperature for 5min, centrifuge at 12000r / min, 2-8℃ for 10min precipitation.
[0030] (3) Add 0.2ml chloroform to every 1ml TRIZOL solution, oscillate for 15sec to mix thoroughly, place at room temperature for 5min, centrifuge at 12000r / min, 2-8°C for 15min.
[0031] (4) Take the supernatant, add 0.5ml of isopropanol to every 1ml of TRIZOL, mix well, and place at room temperature for 10-20min.
[0032] (5) Centrifuge at 12,000 r / min at 2-8°C for 10 minute...
Embodiment 2
[0109] Example 2, Functional verification of honeysuckle p-coumaroyl ester 3'-hydroxylase gene LjC3'H
[0110] 2.1 Construction of prokaryotic expression vector
[0111] 2.1.1 Obtaining the target gene
[0112] (1) Primer design: Design a pair of LjC3'H genes with EcoRI and XhoI restriction sites for PCR amplification.
[0113] P2:5'-G AATTCA TGGCATTCGCTCTCCTC-3'
[0114] EcoRI
[0115] P3:5'-C TCGAGC TAGTAATCCACTGGGACACG-3'
[0116] wxya
[0117] (2) PCR reaction system:
[0118]
[0119] (3) The PCR reaction conditions are
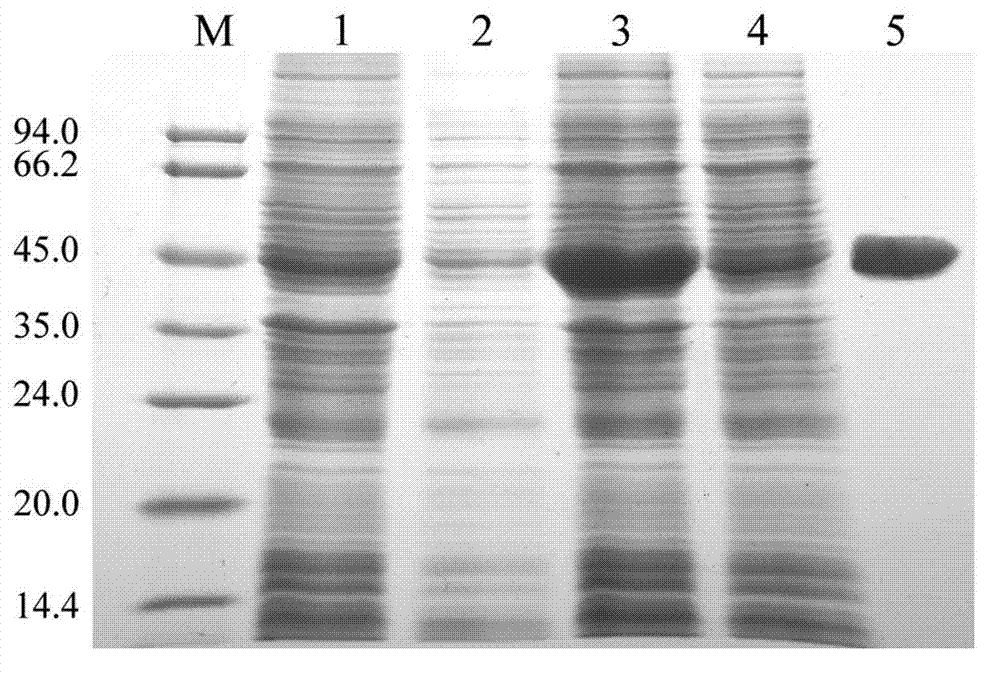
[0120] 94°C for 5min; 94°C for 30sec, 60°C for 30sec, 72°C for 2min, 35 cycles; finally 72°C extension for 7min. The PCR products were recovered by electrophoresis and used in subsequent reactions. PCR product electrophoresis as figure 1 shown.
[0121] 2.1.2 Construction steps of prokaryotic expression vector
[0122] The prokaryotic expression vector pET28a (purchased from Treasure Bioengineering Co., Ltd.) was introduced, and the vec...
Embodiment 3
[0149] Example 3, LjC3'H protein induction expression and protein purification of Lonicera japonica
[0150] 3.1 Induced expression of target protein
[0151] Escherichia coli BL21 single clones containing recombinant expression plasmids pETLjC3'H and pET28a were respectively inserted into 5 mL LB medium containing 50 μg / mL kanamycin, cultured overnight at 37 ° C, diluted 100 times the next day and continued to culture to OD 600 When it was 0.6, IPTG (Isopropyl-beta-D-thio galactoside) with a final concentration of 0.5mmol / L was added, and the expression was induced at 28°C for 4h. Bacterial cultures were harvested by centrifugation, resuspended in phosphate buffer (50mmol / L PBS, pH 7.2), crushed on ice by ultrasonication, and detected by SDS-PAGE for total and soluble proteins.
[0152] 3.2 Purification of target protein
[0153] Pass the supernatant over chelated Ni 2+ Nickel Sepharose column (5ml), and then reduce the concentration of urea by gradient (so that the concen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com