Synthesis method for 5-bromo-ethyl levulinate
A technology of ethyl bromolevulinate and ethyl acetoacetate, which is applied in the field of synthesis of the pharmaceutical intermediate 5-ethyl bromolevulinate, can solve the problems of difficult industrial production, high economic cost, and many by-products, and achieve The effect of high conversion rate, simple synthesis method and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
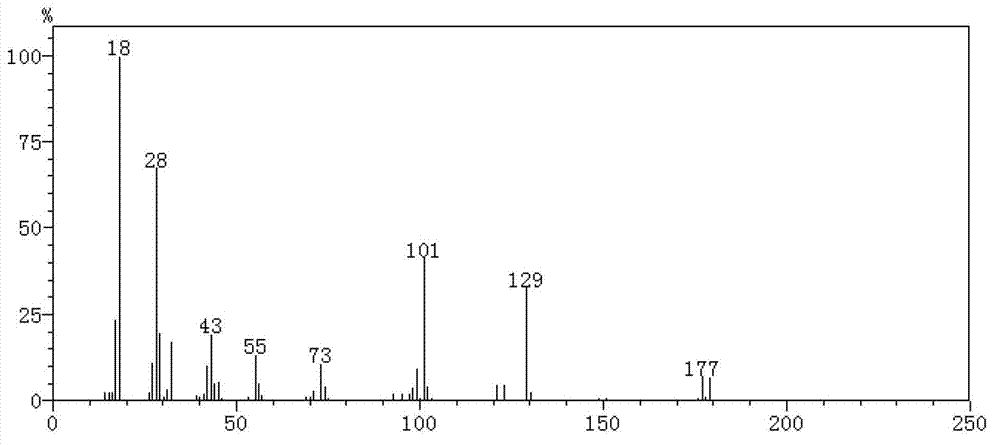
[0021] Preparation of ethyl 5-aminolevulinate intermediate product, ethyl 5-bromolevulinate.
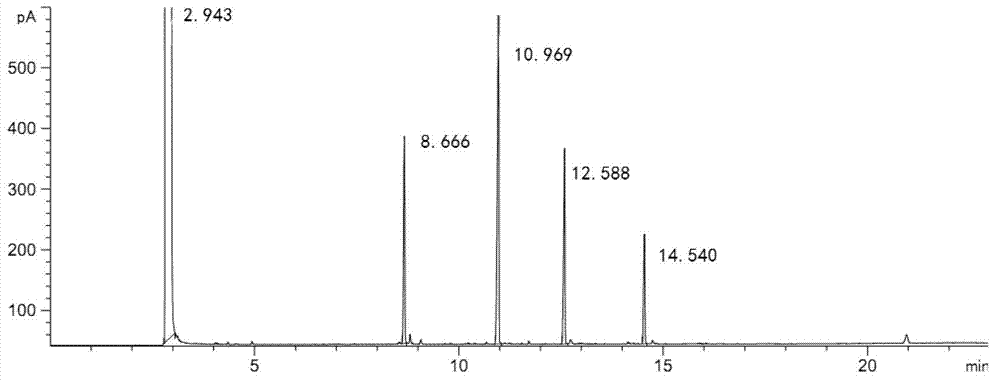
[0022] Mix ethyl levulinate (14.4g) with 100ml of absolute ethanol, add 16g of liquid bromine dropwise to the medium for 2 hours, react at 35°C for 4 hours under stirring conditions, and obtain a light yellow transparent liquid. Take 1ml of the reaction solution, and detect the content of ethyl 5-bromolevulinate by gas chromatography. The selectivity of ethyl 5-bromolevulinate can reach 60%.
[0023] After taking 200g of the reaction solution and distilling ethanol under reduced pressure at 45°C, add 200ml of anhydrous ether and 10ml of water respectively, let stand to separate the water phase, and use a large amount of saturated Na 2 CO 3 solution to wash the organic phase, then anhydrous MgSO 4 After drying and distilling off the ether, the brominated mixture was obtained, dissolved in a mixed solvent of ether and cyclohexane (volume ratio: 1:1), cooled to crystallize, and then ...
Embodiment 2
[0025] Preparation of ethyl 5-aminolevulinate intermediate product, ethyl 5-bromolevulinate.
[0026] Mix ethyl levulinate (14.4g) with 50ml of absolute ethanol, add 16g of liquid bromine dropwise to the medium for 2 hours, and react at 35°C for 8 hours under stirring conditions to obtain a light yellow transparent liquid. Take 1ml of the reaction solution, and detect the content of ethyl 5-bromolevulinate by gas chromatography. The selectivity of ethyl 5-bromolevulinate reached 47.4%.
[0027] Take 300g of the reaction solution and distill ethanol under reduced pressure at 45°C, add 300ml of anhydrous ether and 30ml of water respectively, let stand to separate the water phase, and use a large amount of saturated Na 2 CO 3 solution to wash the organic phase, then anhydrous MgSO 4 After drying and distilling off the ether, the brominated mixture was obtained, dissolved in a mixed solvent of ether and cyclohexane (volume ratio: 1:1), cooled to crystallize, and then recrystall...
Embodiment 3
[0029] Preparation of ethyl 5-aminolevulinate intermediate product, ethyl 5-bromolevulinate.
[0030] Mix ethyl levulinate (14.4g) with 50ml of absolute ethanol, add 16g of liquid bromine dropwise to the medium for 2 hours, and react at 5°C for 2 hours under stirring conditions to obtain a light yellow transparent liquid. Take 1ml of the reaction solution, and detect the content of ethyl 5-bromolevulinate by gas chromatography. The selectivity of ethyl 5-bromolevulinate reached 30.1%.
[0031] After taking 200g of the reaction solution and distilling ethanol under reduced pressure at 45°C, add 200ml of anhydrous ether and 10ml of water respectively, let stand to separate the water phase, and use a large amount of saturated Na 2 CO 3 solution to wash the organic phase, then anhydrous MgSO 4 After drying and distilling off the ether, the brominated mixture was obtained, dissolved in a mixed solvent of ether and cyclohexane (volume ratio: 1:1), cooled to crystallize, and then ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com