Fluorescence chemical sensor capable of selectively detecting biological sulfhydryl compound, preparation method and application of fluorescence chemical sensor
A technology of chemical sensors and mercapto compounds, which is applied in the field of chemical sensors, can solve the problem of fewer molecules in the sensor, and achieve the effects of high sensitivity, easy control of reaction conditions, and high molecular yield of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] A preparation method and application of a fluorescent chemical sensor capable of selectively detecting biothiol compounds:
[0049] (1) Synthesis of BODIPY main compound:
[0050]①Weigh 8g (66.6mmol) of p-tolualdehyde, add 100mL (1441mmol) of pyrrole, slowly drop 0.5mL (6.7mmol) of trifluoroacetic acid into it during stirring, stir at room temperature for 10 minutes, and distill off pyrrole under reduced pressure A black viscous liquid was obtained, and the residue was subjected to column chromatography to obtain the compound represented by formula (B):
[0051]
[0052] ②Dissolve 2g (8.5mmol) of the compound shown in formula B in 100mL tetrahydrofuran, pass N 2 , cooled to -78°C, added 2.3g (17.2mmol) N-chlorosuccinimide, stirred at -78°C for 2 hours, then placed in the refrigerator for 18 hours, evaporated the solvent, and the residue Diluted with 100 mL of dichloromethane, washed 3 times with distilled water, dried the organic layer with anhydrous magnesium sulf...
Embodiment 2
[0072] A preparation method of a fluorescent chemical sensor for selectively detecting biological thiol compounds:
[0073] (1) Synthesis of BODIPY main compound: same as Example 1;
[0074] (2) Weigh 100mg (0.18mmol) of BODIPY main compound A and dissolve it in acetonitrile, pass through N 2 , add 125 mg (0.9 mmol) p-nitrophenol and 250 μL (1.8 mmol) triethylamine, N 2 Reflux for 5 hours under protection. The solvent was distilled off under reduced pressure, and the residue was subjected to column chromatography to obtain the fluorescent compound shown in formula (II).
[0075] Light sensor molecules:
[0076]
[0077] Prepare 1 mmol / L acetonitrile solution of the compound represented by formula (II).
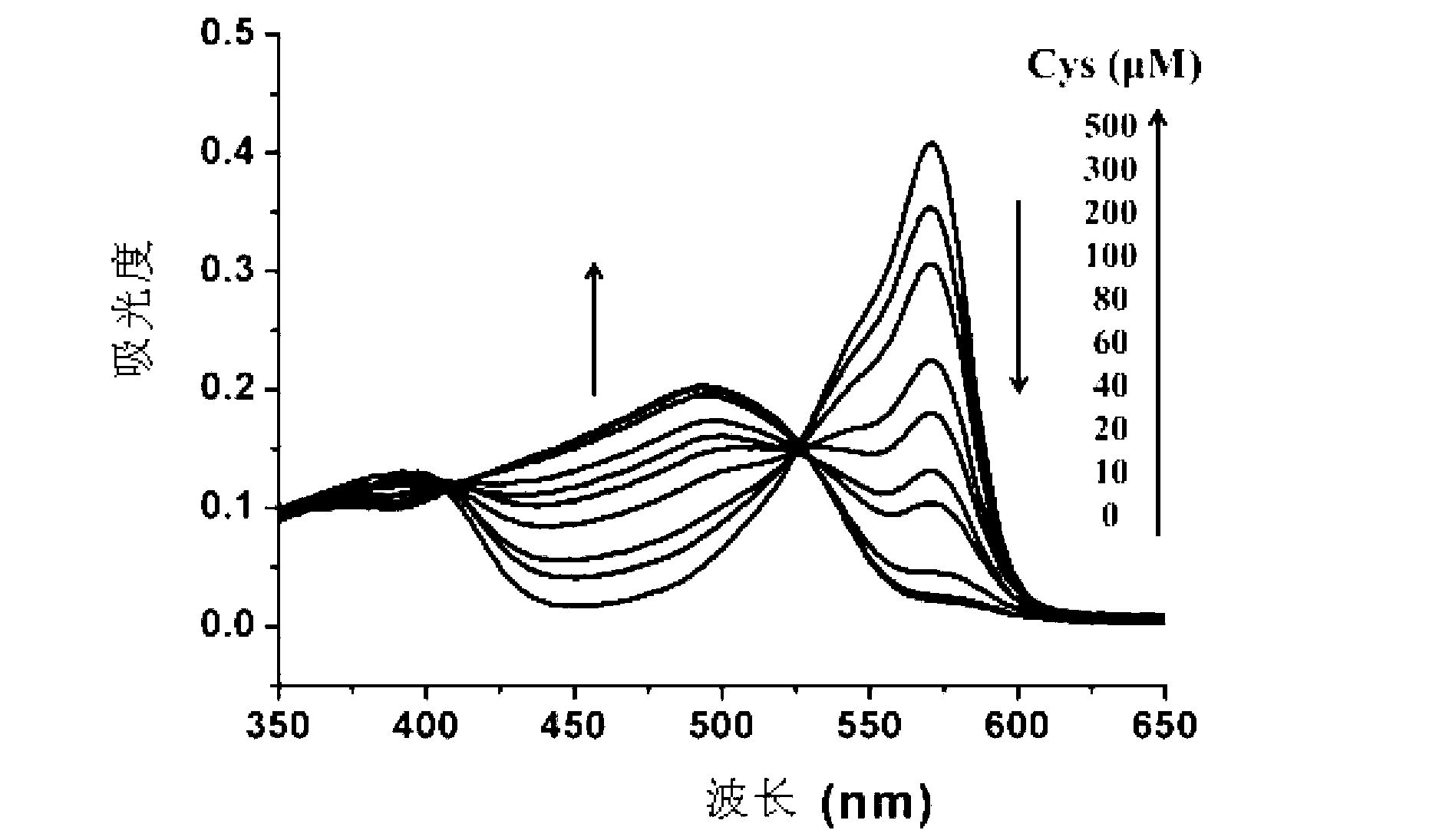
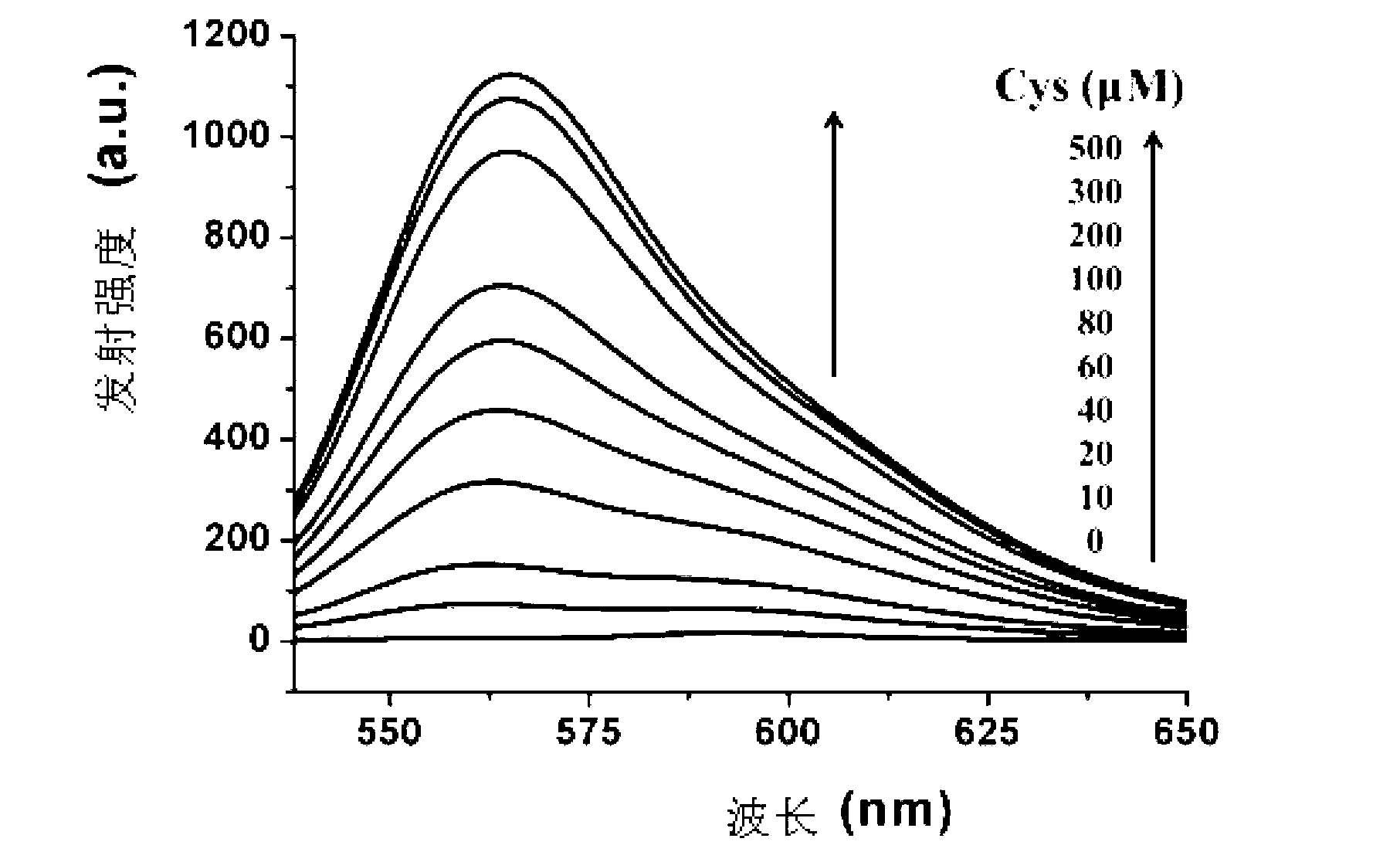
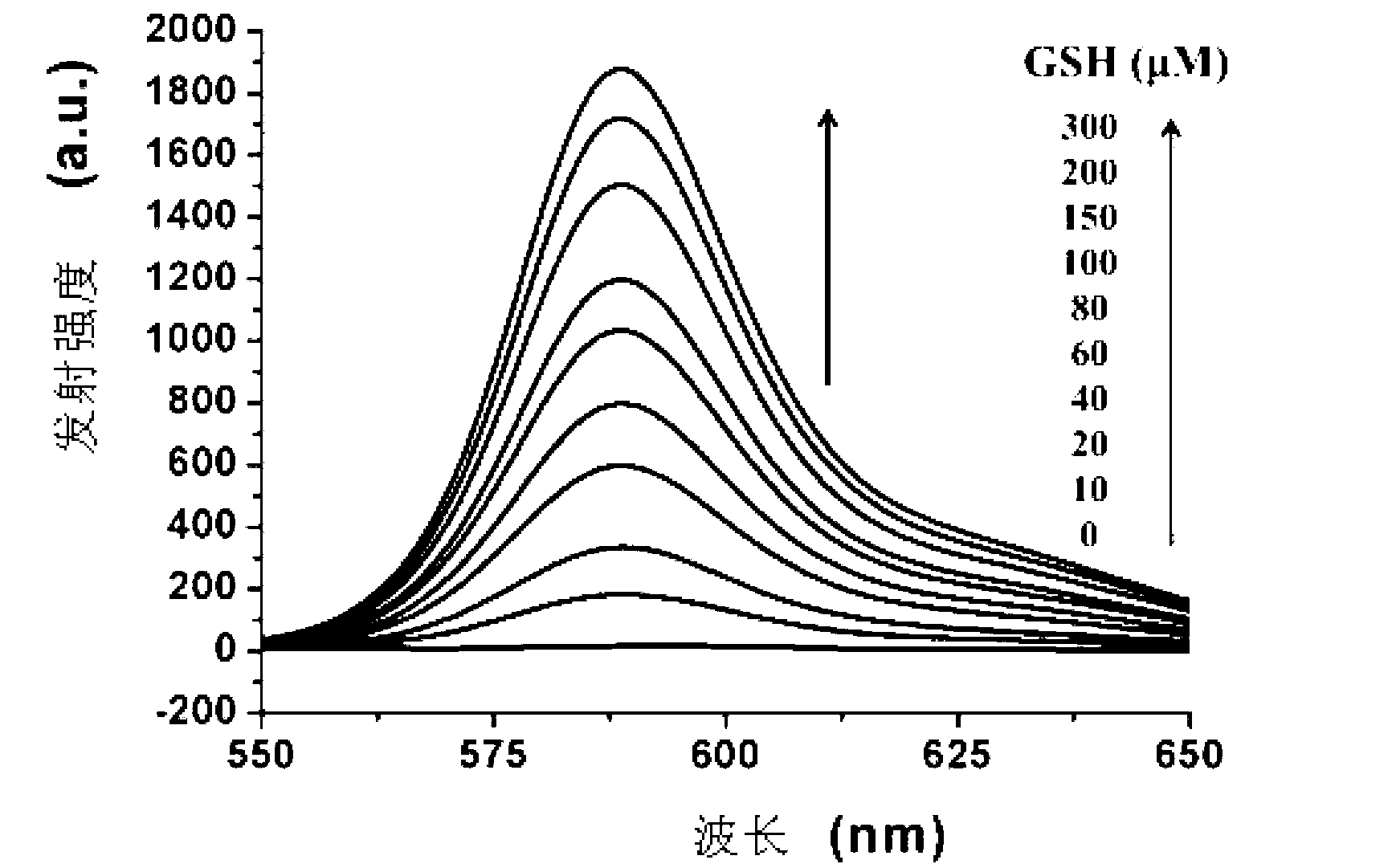
[0078] Cys absorption and emission spectrum test of the compound shown in formula (I): Use acetonitrile / HEPES buffer solution (v / v=2 / 8, 20mM, pH=7.4) to prepare 1mmol / L Cys, GSH and Hcy solutions and blank solution. Take 2 mL of the above-mentioned different solutions...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com