Method for preparing key intermediate of beta-methylcarbapenem antibiotic
A technology of methyl carbapenem and antibiotics, applied in the field of organic synthesis and preparative chemistry, which can solve the problems of long synthetic route, low yield, and limited industrial application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
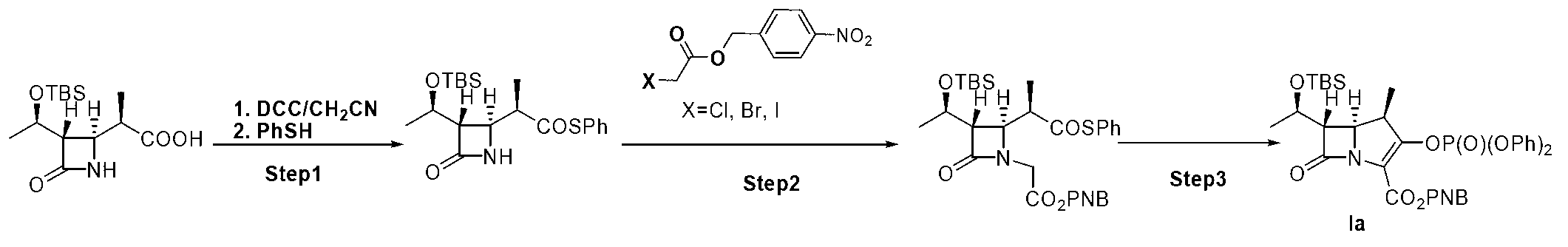
[0026] Embodiment 1: a kind of method for preparing the key intermediate of β-methyl carbapenem antibiotics is characterized in that the specific preparation steps are as follows:
[0027] (1) Condensation reaction: Add 50.0kg β-methyl-ADC-8 and 500.0 L acetonitrile to a 2000 L reactor, cool the system down to -15~-10°C, and add 37.6kg N to the system at this temperature , N'-dicyclohexylcarbodiimide, after the addition, the system was stirred at -15~-10°C for 60 minutes, slowly added 20.1kg of thiophenol to the system, and the temperature was returned to 15~25°C after dropping , and kept stirring at 15-25°C until β-methyl-ADC-8 completely disappeared, filtered with suction, concentrated, washed, concentrated the washed organic phase, and crystallized with 500L n-heptane to obtain 58.8kg of compound , purity 99.3%, yield 90.2%;
[0028] (2) Alkylation reaction: Add 58.8kg of compound into a 2000L reactor , 45.0kg compound , and 588.0L tetrahydrofuran, lower the temperatu...
Embodiment 2
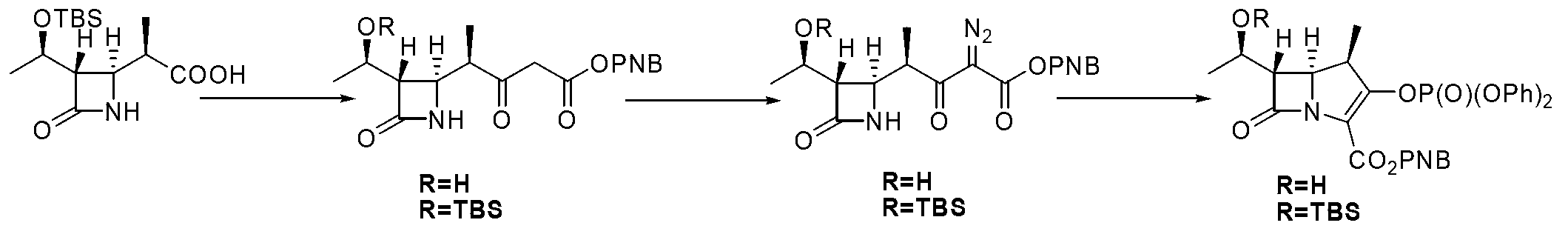
[0030] Embodiment 2: a kind of method for preparing the key intermediate of β-methyl carbapenem antibiotics is characterized in that the specific preparation steps are as follows:
[0031] (1) Condensation reaction: 20.0kg of β-methyl-ADC-8, 100.0 L of methanol, lower the temperature of the system to -10~-5°C, and add 13.6kg of N,N'-bicyclic to the system at this temperature Hexylcarbodiimide, after the addition, the system was stirred at -10~-5°C for 30 minutes, and 7.3kg of thiophenol was slowly added dropwise to the system. Keep stirring at ℃ until β-methyl-ADC-8 disappears completely, filter with suction, concentrate, wash, concentrate the washed organic phase, and crystallize with 100L n-heptane to obtain 22.7kg of compound , purity 90.5%, yield 87.0%;
[0032] (2) Alkylation reaction: Add 22.7kg of compound into a 1000L reactor , 16.4kg compound , and 227L of 1,4-dioxane, lower the temperature to -35~-40°C, add 1.5 kg of NaH into the system at this temperature, and...
Embodiment 3
[0034] Embodiment 3: a kind of method for preparing the key intermediate of β-methyl carbapenem antibiotics is characterized in that the specific preparation steps are as follows:
[0035] (1) Condensation reaction: Add 40.0kg β-methyl-ADC-8 and 600.0 L ethanol to a 2000 L reactor, cool the system down to -20~-15°C, and add 41.0kg N to the system at this temperature , N'-dicyclohexylcarbodiimide, after the addition, the system was stirred at -20~-15°C for 60 minutes, and 21.9kg of thiophenol was slowly added dropwise to the system, and the temperature was returned to 15~25°C after the addition , and kept stirring at 15-25°C until β-methyl-ADC-8 completely disappeared, filtered with suction, concentrated, washed, concentrated the washed organic phase, and crystallized with 600L n-heptane to obtain 44.3kg of compound , purity 92.3%, yield 85.0%;
[0036] (2) Alkylation reaction: Add 44.3kg of compound into a 3000L reactor , 46.2kg compound , and 1107.5L of 1,4-dioxane, low...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com