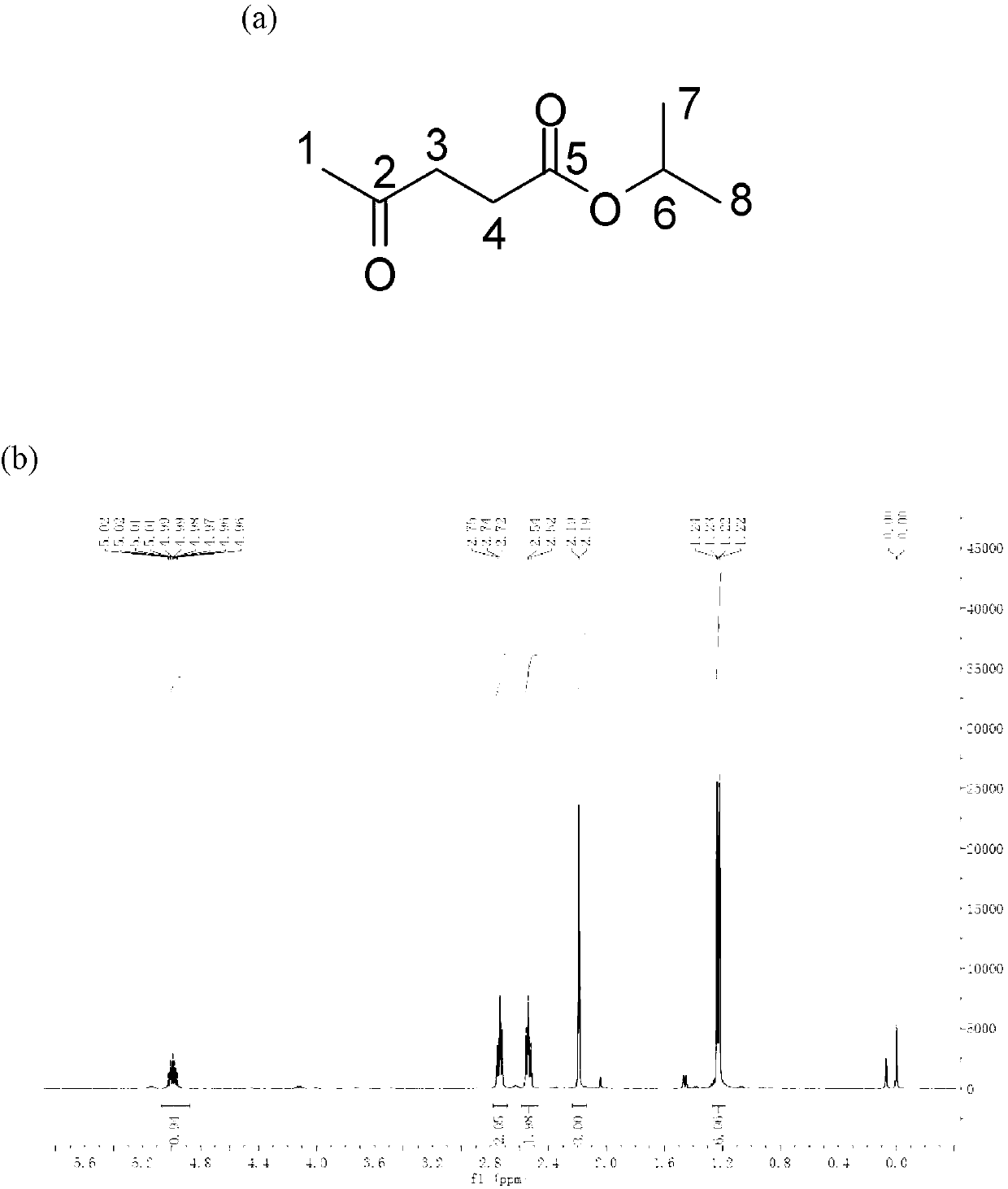
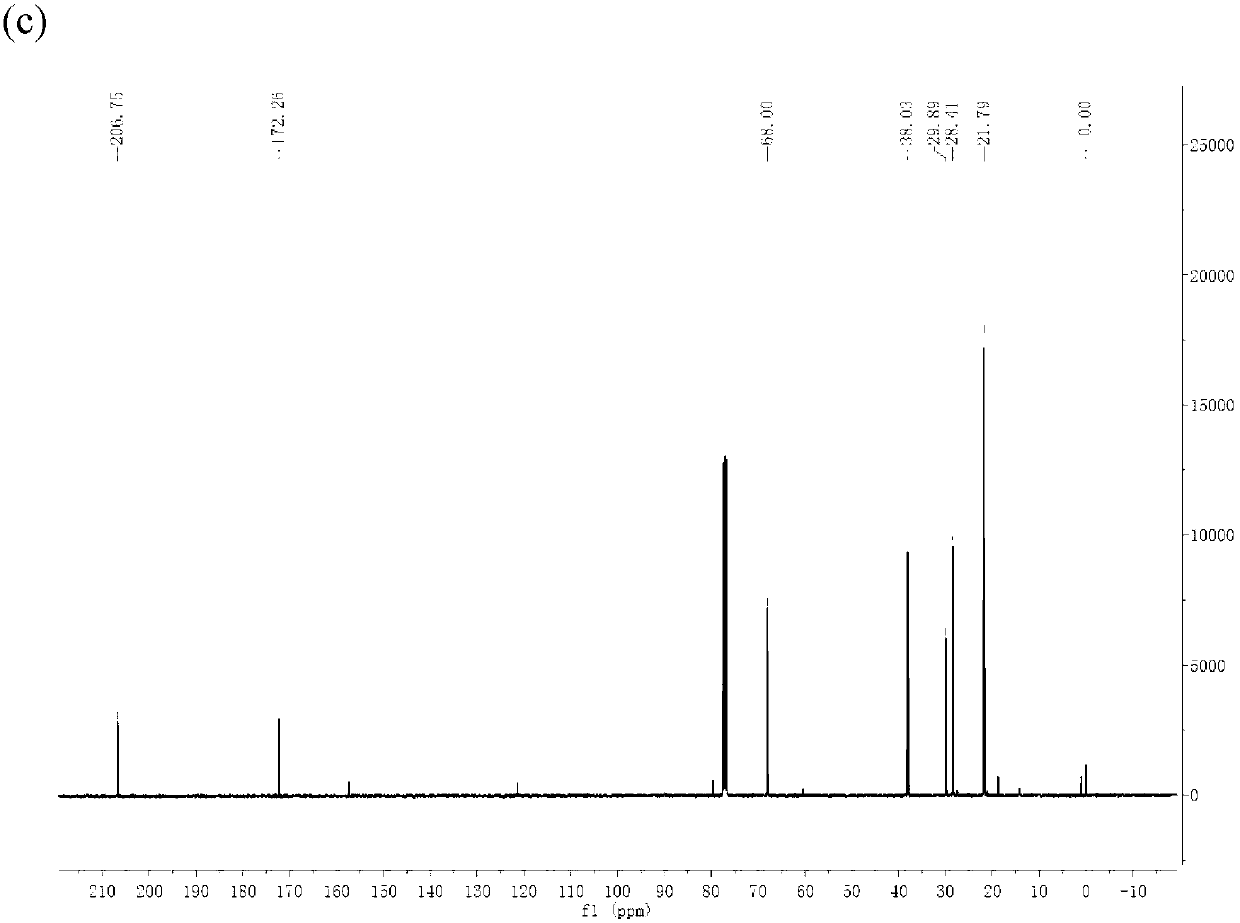
Method for preparing gamma-valerolactone with high selectivity under mild condition
A high-selectivity, valerolactone technology, applied in organic chemistry and other directions, can solve problems such as high energy consumption, environmental pollution, and long reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Use a dry round-bottomed flask with a branch tube under an inert gas N 2 Add 10mmol ethyl levulinate and 20ml isopropanol under protection, then add homemade or commercially available wet weight 1g Raney Ni catalyst under inert gas (Raney Ni can be purchased from reagent companies such as Aldrich), all operations are under inert gas N 2 Under a protective atmosphere, the flask was closed and reacted at room temperature for 9 hours, and the GVL product was obtained through gas chromatography detection, and a transparent and clear GVL liquid product was obtained through vacuum distillation, with a total yield of 99%.
Embodiment 2
[0065] Put 10mmol ethyl levulinate and 20ml isopropanol into a dry round-bottomed flask with a branch pipe, then add homemade or commercially available 1g Raney Ni catalyst (wet weight), all operations without inert gas protection , reacted at room temperature for 9 hours, and detected by gas chromatography to obtain the GVL product, and obtained a transparent and clear GVL liquid product through vacuum distillation, and the total yield was 70%.
Embodiment 3
[0067] Use a dry round-bottomed flask with a branch tube under an inert gas N 2 Add 10mmol ethyl levulinate and 20ml isopropanol under protection, then add homemade or commercially available catalyst with a wet weight of 0.5g Raney Ni under inert gas, all operations are under inert gas N 2 Under a protective atmosphere, the flask was closed and reacted at room temperature for 9 hours, and the GVL product was obtained through gas chromatography detection, and a transparent and clear GVL liquid product was obtained through vacuum distillation, with a total yield of 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com