Preparation method of cathode material Li2Mn1-x-yCoxNiySiO4 for lithium ion battery
A cathode material, lithium ion technology, applied in the field of new energy materials, can solve the problem of low electrical conductivity of materials, and achieve the effect of increasing capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
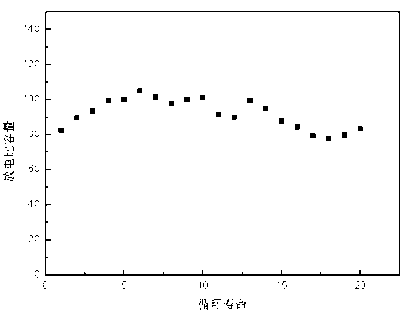
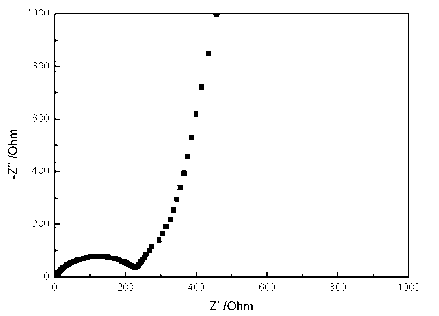
Image
Examples
Embodiment 1
[0024] (1) Li 2 mn 0.2 co 0.3 Ni 0.5 SiO 4 Lithium acetate, manganese acetate, cobalt acetate, nickel acetate and tetraethylorthosilicate were mixed in 45 mL of absolute ethanol according to the molar ratio of 0.02 mol: 0.002 mol: 0.003 mol: 0.005 mol: 0.01 mol, so that Li: Mn : Co: Ni: The molar ratio of Si is 2: 0.2: 0.3: 0.5: 1, then add 0.01 mol of catalyst glacial acetic acid, and magnetically stir to make the solution evenly mixed. (2) Transfer the uniformly mixed raw materials into a 100 mL polytetrafluoroethylene tank, and react in a constant temperature oven at 80 °C for 30 h to obtain a wet gel, and dry the gel-like material in a blast drying oven at 60 °C After drying, a xerogel is obtained. (3) The obtained xerogel was ground into powder in an agate mortar, ball milled with acetone as dispersant for 6 h, and the acetone was evaporated to dryness to obtain the reaction precursor. The precursor was compressed at 6 MPa and calcined at 600 °C for 10 h in a nitrog...
Embodiment 2
[0028] (1) Li 2 mn 0.2 co 0.4 Ni 0.4 SiO 4Lithium acetate, manganese acetate, cobalt acetate, nickel acetate and tetraethylorthosilicate were mixed in 45 mL of absolute ethanol at a molar ratio of 0.02 mol: 0.002 mol: 0.004 mol: 0.004 mol: 0.01 mol, so that Li: Mn : Co: Ni: The molar ratio of Si is 2: 0.2: 0.4: 0.4: 1, then add 0.01 mol of catalyst glacial acetic acid, and magnetically stir to make the solution evenly mixed. (2) Transfer the uniformly mixed raw materials into a 100 mL polytetrafluoroethylene tank, react in a constant temperature oven at 100 °C for 20 h, and obtain a wet gel, and dry the gel-like material in a blast drying oven at 60 °C After drying, a xerogel is obtained. (3) The obtained xerogel was ground into powder in an agate mortar, ball milled with acetone as dispersant for 6 h, and the acetone was evaporated to dryness to obtain the reaction precursor. The precursor was compressed at 6 MPa and calcined at 600 °C for 15 h in a nitrogen atmosphere ...
Embodiment 3
[0030] (1) Li 2 mn 1 / 3 co 1 / 3 Ni 1 / 3 SiO 4 Lithium acetate, manganese acetate, cobalt acetate, nickel acetate and tetraethylorthosilicate were mixed in 45 mL of absolute ethanol at a molar ratio of 0.02 mol: 0.0033 mol: 0.0033 mol: 0.0033 mol: 0.01 mol, so that Li: Mn : Co: Ni: The molar ratio of Si is 2: 0.33: 0.33: 0.33: 1, then add 0.02 mol of catalyst glacial acetic acid, and magnetically stir to make the solution evenly mixed. (2) Transfer the uniformly mixed raw materials into a 100 mL polytetrafluoroethylene tank, react in a constant temperature oven at 100 °C for 25 h to obtain a wet gel, and dry the gel-like material in a blast drying oven at 60 °C After drying, a xerogel is obtained. (3) The obtained xerogel was ground into powder in an agate mortar, ball milled with acetone as dispersant for 6 h, and the acetone was evaporated to dryness to obtain the reaction precursor. The precursor was compressed at 8 MPa and calcined at 700 °C for 10 h in a nitrogen atmosp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com