Preparation method for rare earth element-doped titanium dioxide nano material
A rare earth element and titanium dioxide technology, applied in the direction of titanium dioxide, titanium oxide/hydroxide, nanotechnology, etc., can solve the problems of inconspicuous photocatalytic activity, low ratio of rare earth atoms, low doping utilization rate of rare earth elements, etc. And the effect of simple process, wide adjustable range of parameters, and high performance of photocatalytic decomposition of organic pollutants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
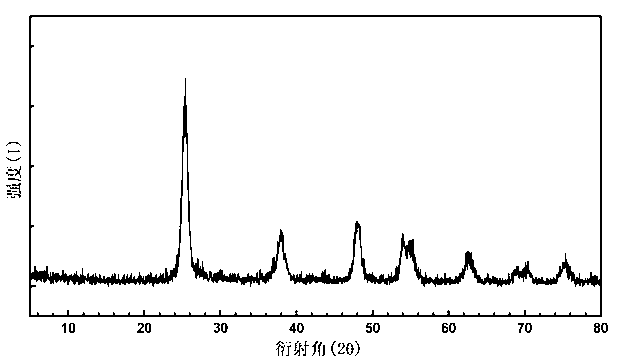
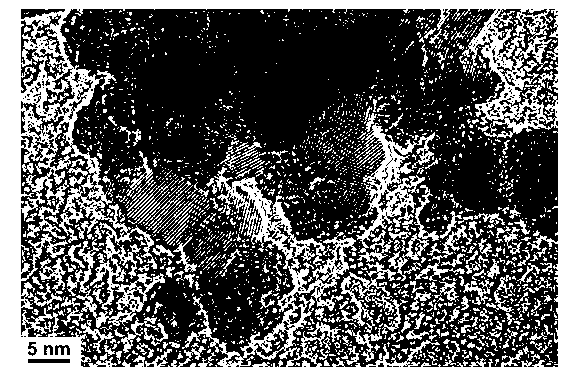
Image
Examples
Embodiment 1
[0020] Add 1 g of urea and 0.5 g of lanthanum nitrate hexahydrate sequentially into 80 ml of absolute ethanol. After stirring to dissolve, add 12 ml of isopropyl titanate and stir evenly. Then, 7 ml of deionized water was added dropwise to the above system while stirring until a transparent gel was formed. The above gel was put into a Teflon-lined stainless steel autoclave for hydrothermal reaction at 170 °C for 48 h. After the reaction, the precipitated product is washed with deionized water until the pH of the washing liquid is neutral, and then dried to obtain the target rare earth element-doped titanium dioxide nanomaterial.
[0021] The obtained rare earth-doped titanium dioxide nanometer material is a highly dispersed nanocrystal grain with a particle diameter of about 8-10 nanometers and uniform size. The degradation rate of 0.1 gram of the above product to a 20 mg / L methyl orange solution under 300 watts of ultraviolet light in 40 minutes can reach more than 99%.
Embodiment 2
[0023] Add 0.5 g of urea, 0.3 g of lanthanum nitrate hexahydrate, and 0.02 g of cerium nitrate hexahydrate in sequence in 50 ml of absolute ethanol. After stirring to dissolve, add 8 ml of n-tetrabutyl titanate and stir evenly. Then, 4 ml of deionized water was added dropwise to the above system while stirring until a transparent gel was formed. The above gel was put into a Teflon-lined stainless steel autoclave for hydrothermal reaction at 150 °C for 24 h. After the reaction, the precipitated product is washed with deionized water until the pH of the washing liquid is neutral, and then dried to obtain the target rare earth element-doped titanium dioxide nanomaterial.
[0024] The obtained rare earth-doped titanium dioxide nanometer material is a highly dispersed nanocrystal grain with a particle diameter of about 5-7 nanometers and uniform size. The degradation rate of 0.1 gram of the above-mentioned product to 20 mg / L methyl orange solution reaches more than 99% within 30 ...
Embodiment 3
[0026] Add 1.2 g of urea, 0.1 g of gadolinium nitrate hexahydrate, and 0.05 g of neodymium nitrate hexahydrate in sequence in 100 ml of absolute ethanol. After stirring to dissolve, add 15 ml of tetraethyl titanate and stir well. Then, 8 ml of deionized water was added dropwise to the above system while stirring until a transparent gel was formed. The above gel was put into a Teflon-lined stainless steel autoclave for hydrothermal reaction at 200 °C for 16 h. After the reaction, the precipitated product is washed with deionized water until the pH of the washing liquid is neutral, and then dried to obtain the target rare earth element-doped titanium dioxide nanomaterial.
[0027] The obtained rare earth-doped titanium dioxide nanometer material is a highly dispersed nanocrystal grain with a particle diameter of about 7-9 nanometers and uniform size. The degradation rate of 0.1 gram of the above product to 20 mg / L methyl orange solution under 300 watts of ultraviolet light for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com