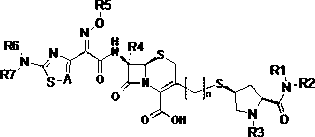
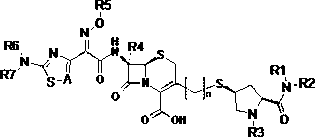
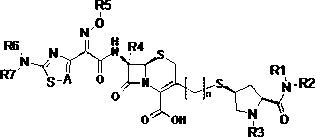
Cephalosporins compound and pharmaceutically acceptable salt thereof
A technology of cephalosporins and compounds, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] (2S,4R)-1-tert-butoxycarbonyl-4-hydroxypyrrolidine-2-carboxylic acid (compound 1)
[0052] Trans-hydroxyproline 40g, dissolved in THF: water (2:1) 500ml, add 10% NaOH solution 170ml, the solution becomes clear; di-tert-butyl dicarbonate 90g dissolved in THF: water (2:1) 250ml, Added to the above solution, stirred overnight at room temperature. THF was distilled off under reduced pressure, 10% KHSO4 solution was adjusted to pH=2, extracted with 400ml×3 ethyl acetate, the extract was washed with water and saturated brine, and dried over anhydrous sodium sulfate. The solvent was evaporated and the crystallization was repeated twice with ethyl acetate to obtain 57.98 g of granular white crystals, mp127-129, and the yield was 82.2%. TOF-MS (254.10[M+Na],485.21[2M+Na])
Embodiment 2
[0054] (2S,4R)-2-Dimethylcarbamoyl-4-methylsulfonyl-1-tert-butoxycarbonylpyrrolidine (compound 2)
[0055] Compound 1 (23.1 g, 0.1 mol) was dissolved in CH at -15 °C 2 Cl 2 200ml, add triethylamine (25.3g, 2.5eq), dropwise add methanesulfonyl chloride (25.5g, 2.2eq), stir for 1 hour, add dimethylamine hydrochloride (16g, 2eq), triethylamine ( 34g), continue to react for 1 hour. To terminate the reaction, the reaction solution was respectively mixed with water, 0.5NHCl solution, water, 5% NaHCO 3 solution, washed with saturated brine, the organic layer was dried with anhydrous magnesium sulfate, concentrated under reduced pressure, crystallized with ethyl acetate-petroleum ether to obtain about 25 g of a solid with crystal luster, and recrystallized with isopropanol to obtain 25.8 g of block Solid, mp 130°C.
Embodiment 3
[0057] (2S,4S)-2-Dimethylcarbamoyl-4-acetylthio-1-tert-butoxycarbonylpyrrolidine (compound 3)
[0058] Compound 2 (6.73g), dissolved in 80ml of DMF:EtOAc (2:3), added 3.42g of potassium thioacetate, heated to 65°C and stirred for 10 hours, the reaction solution was cooled, diluted with 30ml of water, and diluted with 30ml of ethyl acetate ×3 extraction, the extract was washed with 1% sulfuric acid, water, and saturated brine, dried over anhydrous magnesium sulfate, concentrated under reduced pressure, and recrystallized with ethyl acetate-petroleum ether to obtain 5.80 g of crystals, Mp99-101°C, yield 91.62 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com