Medicine capable of promoting secretion of glp-1 and restraining secretion of gip
A technology of inhibitors and medicaments, used in endocrine system diseases, medical preparations containing active ingredients, pharmaceutical formulations, etc., can solve the problems of needing a doctor, unable to completely inhibit secretion, etc., to promote secretion, and achieve excellent human safety. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0039] The agent of the present invention is among antagonistic inhibitors and non-antagonistic inhibitors of sucrose decomposing enzymes, and by using the non-antagonistic inhibitor, it stimulates only L cells in the lower intestinal tract without stimulating K cells in the upper intestinal tract. Stimulates and promotes the secretion of desired GLP-1 and inhibits the secretion of undesired GIP, thus being an extremely useful substance for the treatment of abnormal glucose tolerance or the prevention of diabetes.
[0040] As the non-antagonist inhibitor of the active ingredient of the medicament of the present invention, any conventionally known substance can be used, but from the viewpoint of safety, it is preferably present in nature, and from the viewpoint of food experience, generally Preference is given to plant origin for food use. In addition, when using substances that hardly exist in nature, it is preferable to use those that can be added to foods approved by public ...
Embodiment 1
[0050] [Example 1: Validation test of the effect of using L-arabinose as a non-antagonist inhibitor]
[0051] 【experiment method】
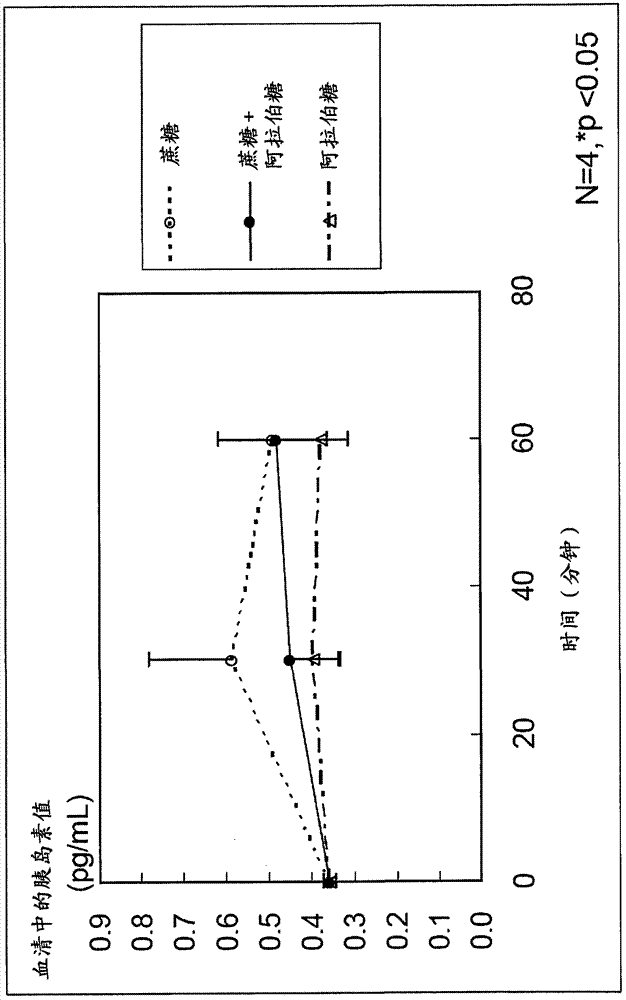
[0052] For the case where sucrose and L-arabinose were administered to rats, the case where sucrose alone (control) was administered to rats, and the case where L-arabinose alone (control) was administered to rats, GLP-1 And the secretion of GIP, check the inhibitory effect of blood sugar and insulin.
[0053] First, 12 SD male rats (4-week-old) acclimated to normal food for 1 week were used, and these were divided into 3 groups of 4 each, and 2.5 g of sucrose / body weight kg+L-arabinose was orally administered to each group. 125 mg / kg body weight (5% relative to sucrose), 2.5 g / kg body weight sucrose, or 125 mg / kg body weight L-arabinose.
[0054] Portal blood and venous blood were collected from each rat before administration, 30 minutes after administration, and 60 minutes after administration. Prepare a blood collection tube filled with DPP-...
Embodiment 2
[0058] [Example 2: Test to check the effect of varying the amount of L-arabinose used]
[0059] 【experiment method】
[0060] The amount of L-arabinose administered to the rats in Example 1 was varied, and changes in the secretion amounts of GLP-1 and GIP were investigated.
[0061] First, using 24 male SD rats (4 weeks old) acclimated to normal food for 1 week, these were divided into 6 groups of 4 each, and 2.5 g of sucrose / body weight kg+L-arabinose was orally administered to each group. 25mg / body weight kg (relative to sucrose 1%), sucrose 2.5g / body weight kg+L-arabinose 62.5mg / body weight kg (relative to sucrose 2.5%), sucrose 2.5g / body weight kg+L-arabinose 100mg / body weight kg ( Relative to sucrose 4%), sucrose 2.5g / body weight kg+L-arabinose 250mg / body weight kg (relative to sucrose 10%), sucrose 2.5g / body weight kg+L-arabinose 500mg / body weight kg (relative to sucrose 20%), Or 2.5g of sucrose / kg of body weight. The post-administration treatment was performed as in E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com