AMPK (Adenosine Monophosphate Activated Protein Kinase) activating agent and application thereof in preparation of medicaments for treating diabetes mellitus and/or diabetic complication
A technology for diabetes and complications, used in drug combinations, urinary system diseases, sexual diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
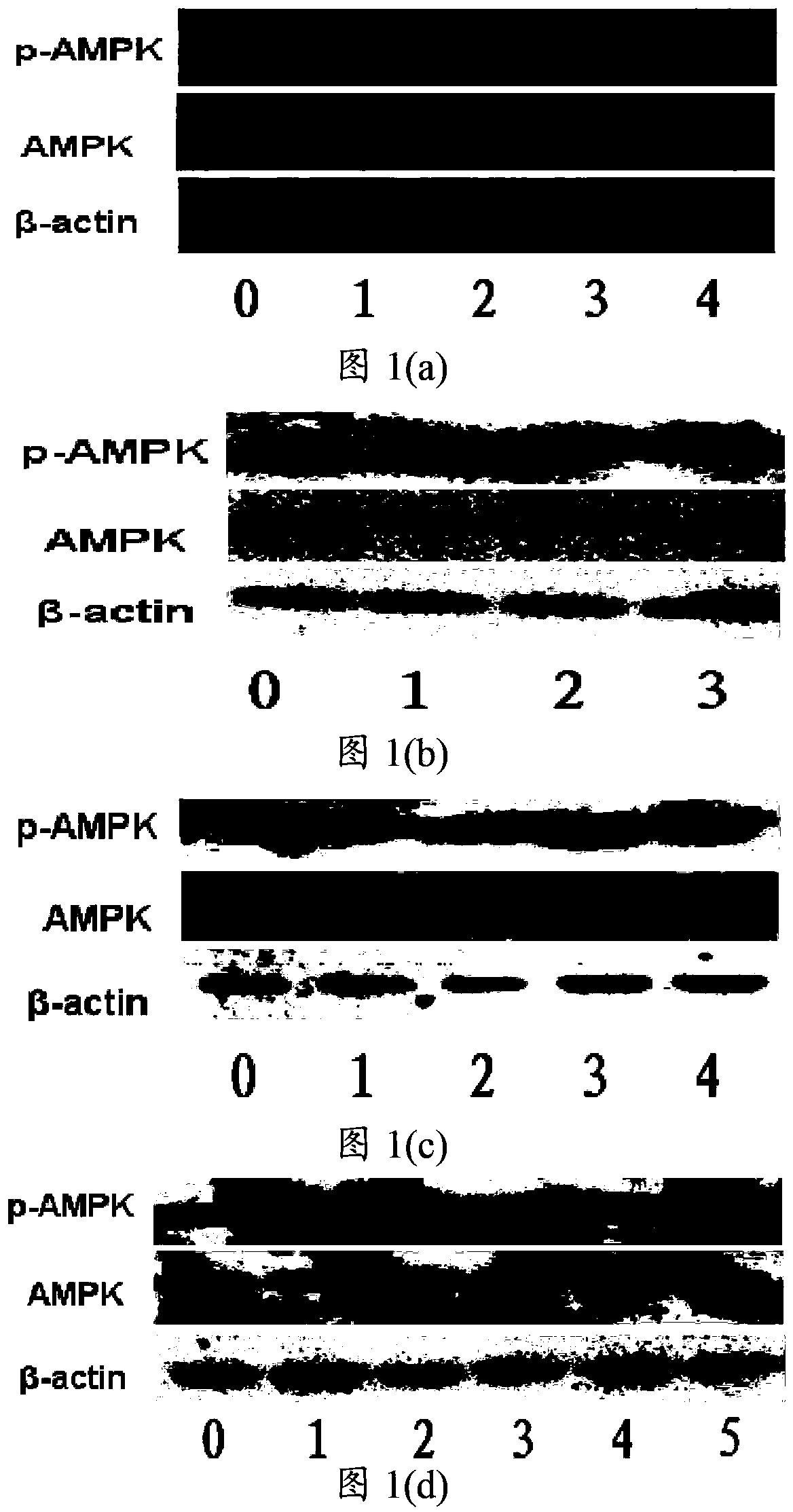
[0079] The compound shown in embodiment 1 formula I is to the activation of macrophage cell AMPK
[0080] Take out the mouse macrophage cells from the liquid nitrogen tank and quickly place them in warm water at 37°C to shake gently to thaw, centrifuge at 1000 rpm for 3 minutes, discard the supernatant, and culture the cells in DMEM with a mass fraction of FBS of 10%. When the coverage rate of the cells in the culture dish reached 60%-70%, subculture was carried out. After the subcultured cell coverage reached 60%-70%, the cells were obtained by centrifugation at 1000 rpm for 3 min, stained with trypan blue, and counted under a microscope with a hemocytometer. Dilute the counted cells to a concentration of 150,000 / mL~200,000 / mL, and spread on a 96-well plate at a concentration of 15,000 / well to 20,000 / well.
[0081] Prepare a 3H-1,2-dithiocyclopentene-3-thione compound solution, weigh the compound shown in formula I, and dissolve it in DMSO so that the final concentration of ...
Embodiment 2
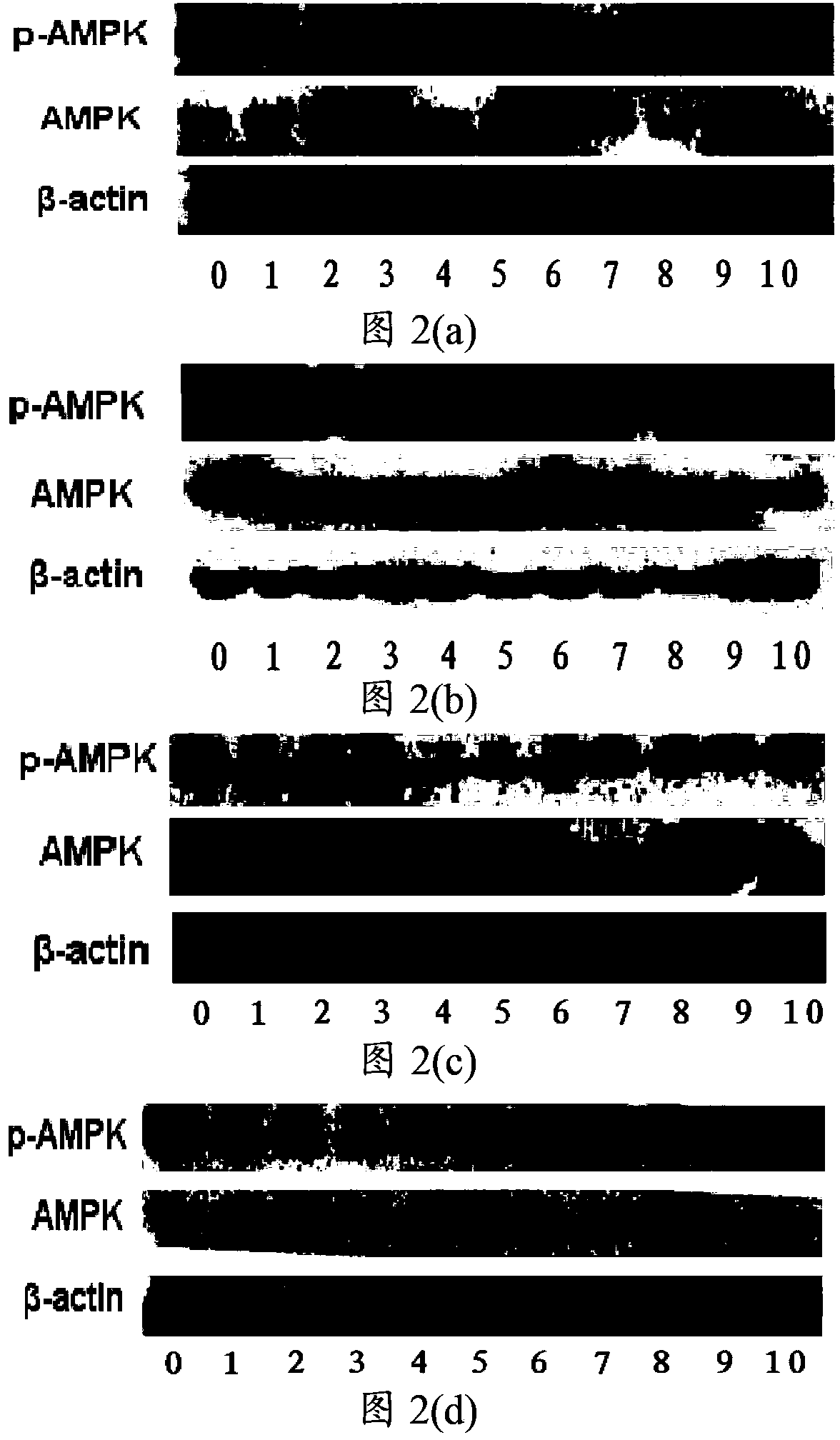
[0088] Example 2 The activating effect of the compound represented by formula I on macrophage AMPK under LPS-induced inflammation conditions
[0089] Take the mouse macrophage cells out of the liquid nitrogen tank and quickly place them in warm water at 37°C and shake gently to thaw, centrifuge at 1000 rpm for 3 minutes, discard the supernatant, and place the cells in DMEM medium with a mass fraction of FBS of 10%. Cultivate and subculture when the cell coverage in the culture dish reaches 60%-70%. After the subcultured cell coverage reached 60%-70%, the cells were obtained by centrifugation at 1000 rpm for 3 min, stained with trypan blue, and counted under a microscope with a hemocytometer. Dilute the counted cells to a concentration of 150,000 / mL~200,000 / mL, and spread on a 96-well plate at a concentration of 15,000 / well to 20,000 / well.
[0090] Prepare a 3H-1,2-dithiocyclopentene-3-thione compound solution, weigh the compound shown in formula I, and dissolve it in DMSO so ...
Embodiment 3
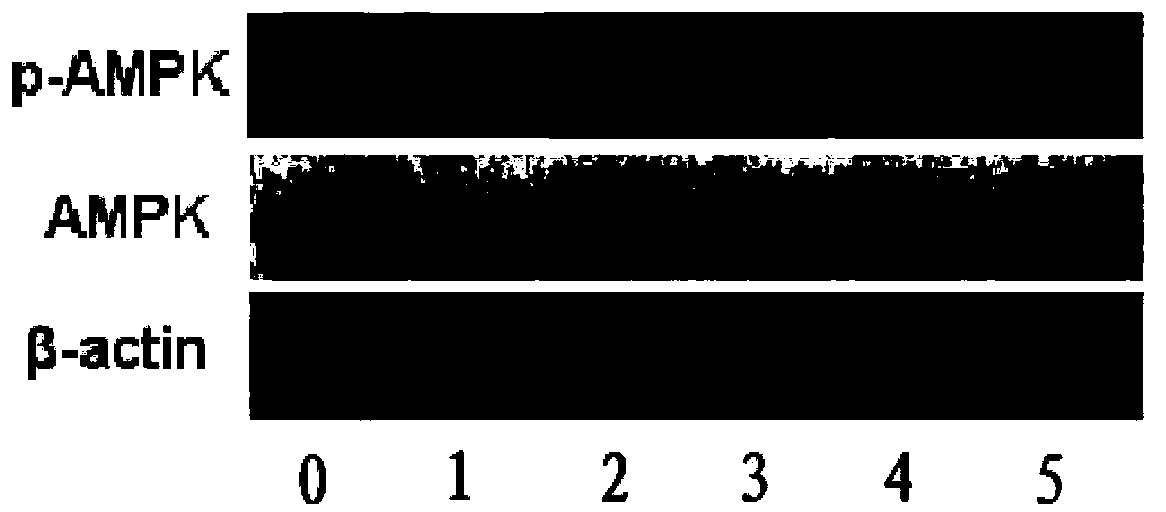
[0099] The activation effect of the compound shown in Example 3 formula I on human cell KEK293T AMPK
[0100] Take the KEK293T cells out of the liquid nitrogen tank and quickly place them in warm water at 37°C to shake gently to thaw, centrifuge at 1000 rpm for 3 minutes, discard the supernatant, and culture the cells in DMEM medium with a mass fraction of FBS of 10%. Subculture was carried out when the coverage rate in the petri dish reached 60%-70%. After the subcultured cell coverage reaches 60%-70%, centrifuge at 1000 rpm for 3 minutes to obtain the cells, stain with trypan blue, count 150,000 / mL~200,000 / mL under a microscope with a hemocytometer, and spread on a 96-well plate , 15,000 / hole to 20,000 / hole.
[0101] Prepare a 3H-1,2-dithiocyclopentene-3-thione compound solution, weigh the compound shown in formula I, and dissolve it in DMSO so that the final concentration of the solution is 50 mmol / L in terms of mass concentration.
[0102]
[0103] Formula I
[0104]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com