Enrofloxacin sustained-release tablets for pets and preparation method for same
A technology of enrofloxacin and sustained-release tablets, which is applied in the field of enrofloxacin sustained-release tablets for pets and its preparation, can solve the problems of excessive fluctuation of blood drug concentration and great influence on the treatment effect, and reduce injuries risk, improve the effect of clinical treatment, and the effect of simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
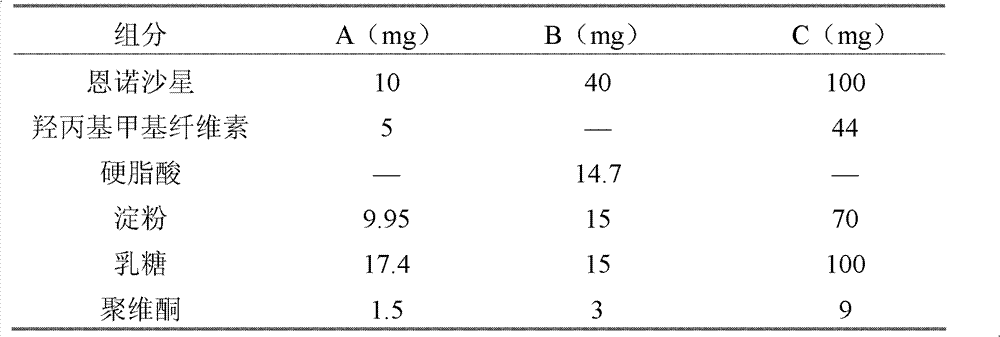
[0019] Party:
[0020]
[0021]
[0022] Preparation:
[0023] A. Grind enrofloxacin, hydroxypropyl methylcellulose or stearic acid, starch, and lactose respectively, pass through a 100-mesh sieve, mix well, and set aside for later use;
[0024] B, adding povidone in 80% aqueous ethanol solution to dissolve, as adhesive, for subsequent use;
[0025] C. Add the prepared adhesive to the mixed raw and auxiliary materials, granulate, dry to ensure that the moisture content is below 5%, and pass through a 20-mesh sieve;
[0026] D. Grind the remaining auxiliary materials, pass through a 100-mesh sieve, add to the prepared granules, mix evenly, and press into tablets.
Embodiment 2
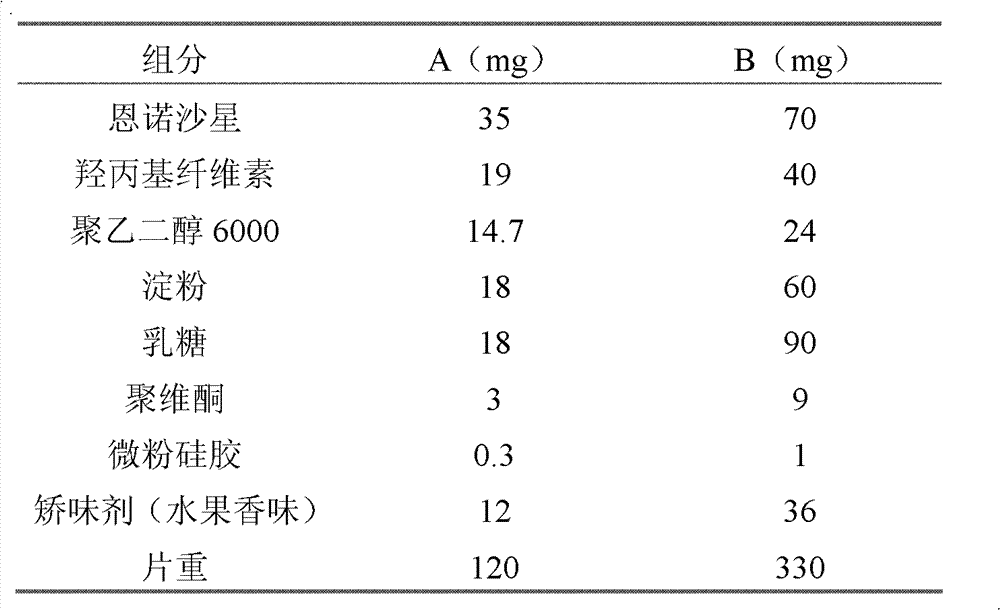
[0028] Party:
[0029]
[0030] Preparation:
[0031] A. Grind enrofloxacin, hydroxypropyl cellulose, polyethylene glycol 6000, starch and lactose respectively, pass through a 100-mesh sieve, mix well, and set aside for later use;
[0032] B, adding povidone in 80% aqueous ethanol solution to dissolve, as adhesive, for subsequent use;
[0033] C. Take 75% of the mixed raw and auxiliary materials and add the prepared binder, granulate, and dry to ensure that the moisture content is below 5% and pass through a 20-mesh sieve;
[0034] D. After passing through a 100-mesh sieve, the remaining mixed raw and auxiliary materials are added to the prepared granules, mixed evenly, and pressed into tablets.
Embodiment 3
[0035] Example 3 Stability test
[0036] 1 Materials and methods
[0037] 1.1 Material test drug: Enrofloxacin sustained-release tablets prepared according to Example 1-A, Example 1-B, Example 1-C, Example 2-A, Example 2-B respectively; control Medicine: According to the patent (CN101190194A, published on June 4, 2008), self-made enrofloxacin chewable tablets for dogs and cats, the content is 5mg.
[0038] 1.2 Methods Take 25 tablets of the test drug and the reference drug respectively. According to the "Technical Specification for Stability Test of Veterinary Drugs (Trial)", the accelerated test of the drug was carried out for six months. Sampling for content determination.
[0039] 2 Test results
[0040] Example 2 Drug stability test results (n=4)
[0041]
[0042] The results showed that the drug stability of the enrofloxacin sustained-release tablets prepared in Example 1-A, Example 1-B, Example 1-C, Example 2-A, Example 2-B was significantly better than The stabi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com