Method for separating and purifying impurities in sodium tanshinone IIA sulfonate crude drug
A separation and purification technology of sodium sulfonate, which is applied in the field of medicine, can solve problems such as excessive content difference, irreversible adsorption, and low impurity content, and achieve high recovery rate, large preparation volume, and high separation efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
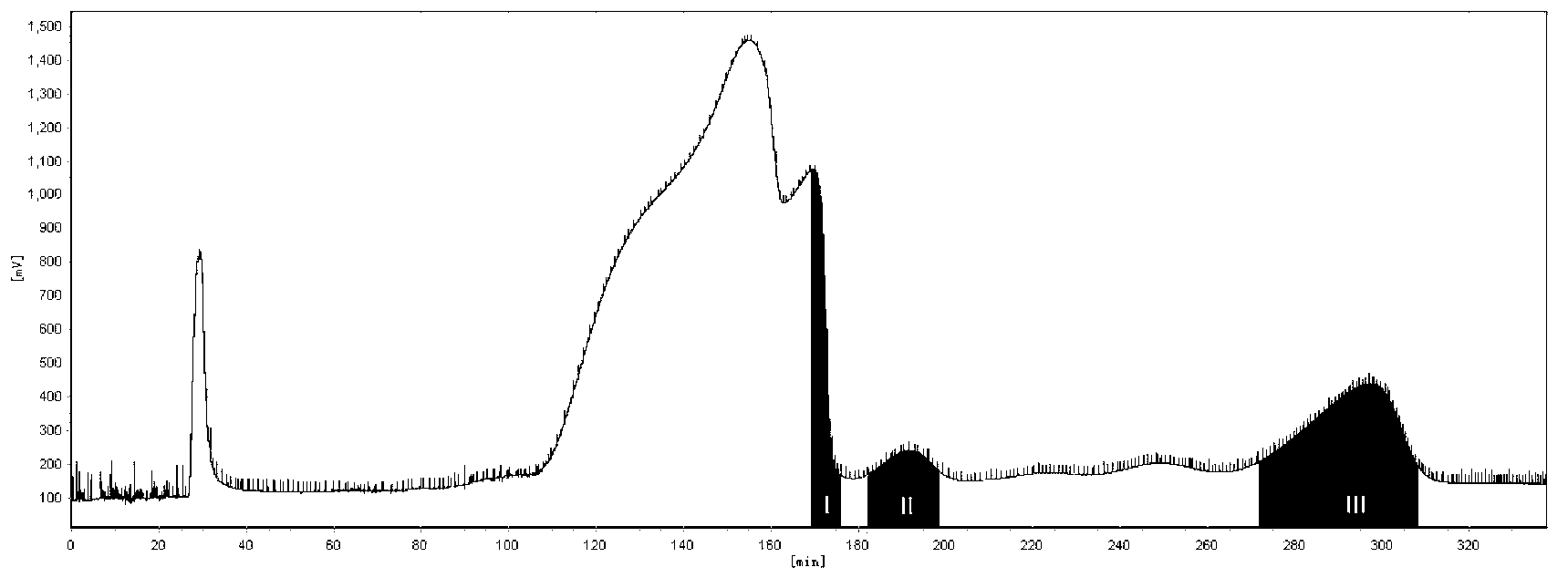
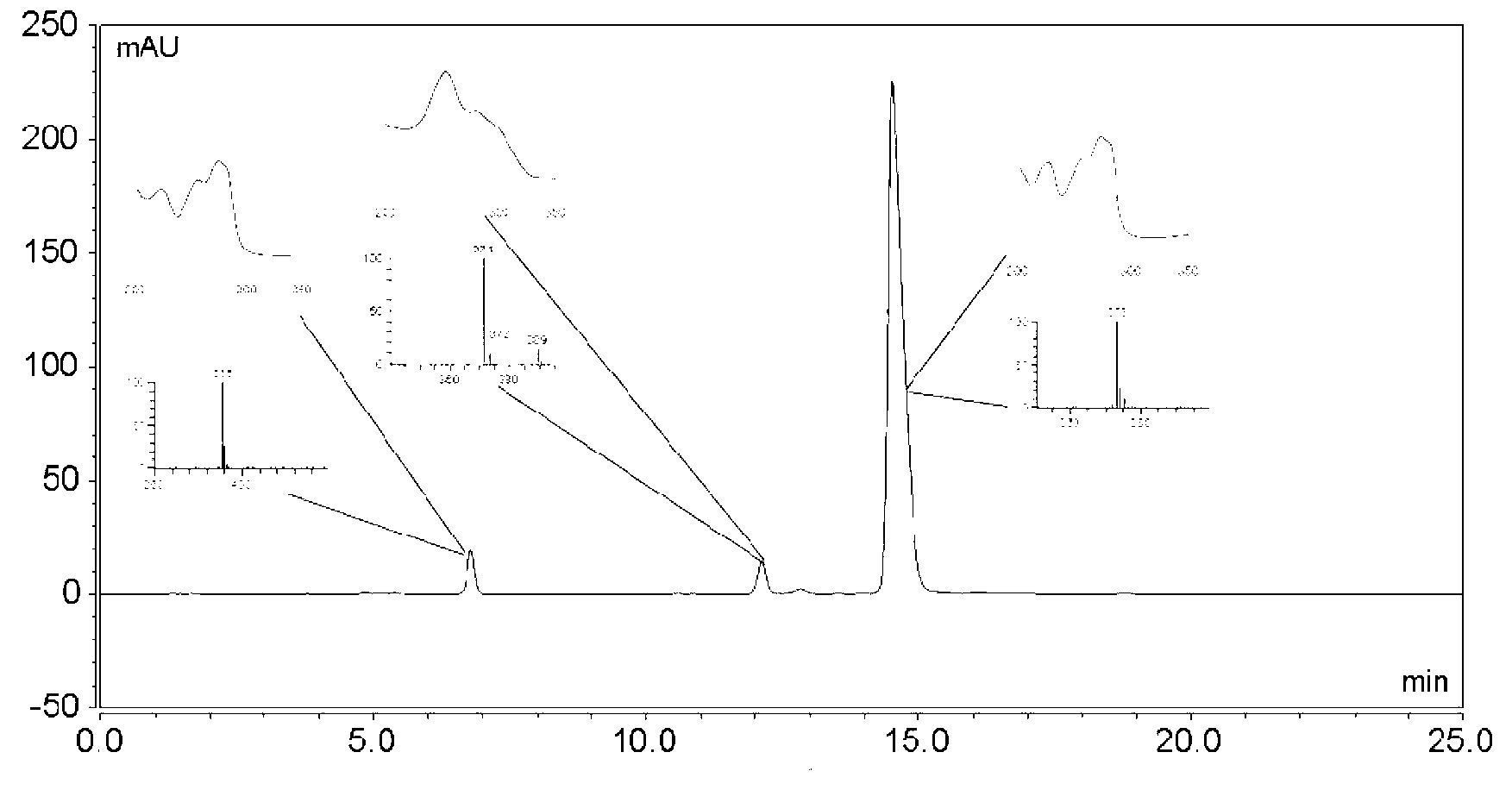
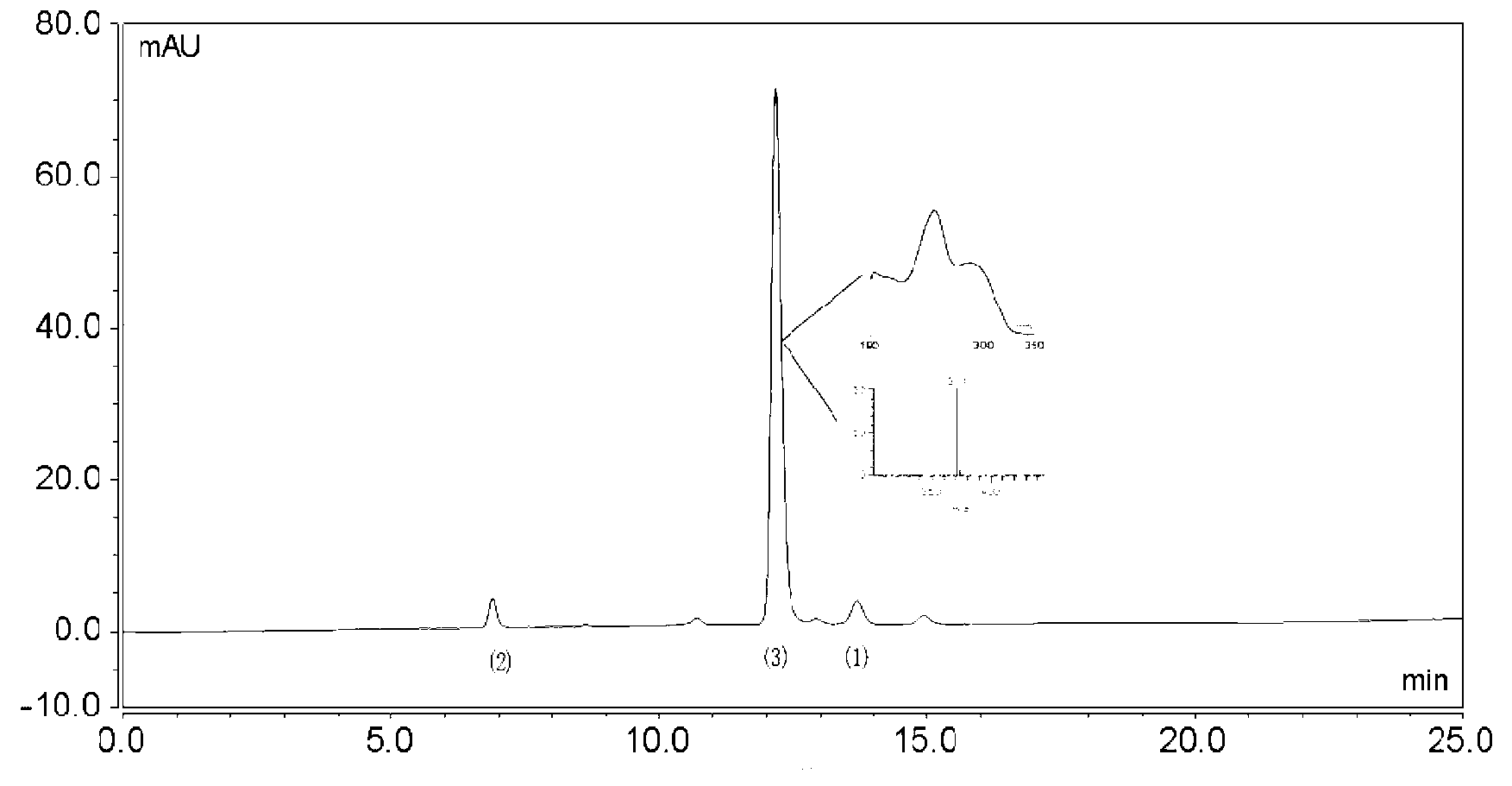
[0022] High-speed countercurrent chromatography (Shenzhen Tongtian Biochemical Co., Ltd.) was used to separate and purify three main impurities from tanshinone IIA sodium sulfonate raw material drug: the solvent system that constitutes the high-speed countercurrent chromatography stationary phase and mobile phase is chloroform: n-butanol: Methanol: 0.5% saturated ammonium acetate aqueous solution = 17: 0.3: 14: 9 (volume ratio). After fully shaking in the separatory funnel, the layers were statically separated, the upper phase was the stationary phase, and the lower phase was the mobile phase. First make the countercurrent chromatographic column full of stationary phase, then make the host rotate, then pump the mobile phase, the volume of the countercurrent chromatographic column is 300ml, the retention rate of the stationary phase is 75%, the flow rate is 2.0ml / min, the rotating speed is 950rpm, and the detection wavelength is 280nm. Dissolve 100mg of tanshinone IIA sodium su...
Embodiment 2
[0026]According to the high-speed countercurrent chromatography operation steps of Example 1, three main impurities are separated and purified from the tanshinone IIA sodium sulfonate bulk drug: the solvent system that constitutes the high-speed countercurrent chromatography stationary phase and mobile phase is chloroform: n-butanol: methanol ︰0.5% saturated ammonium acetate aqueous solution=18︰0.2︰15︰8. The methods and steps of separation and purification, purity detection and structural identification of the three impurities are the same as in Example 1. The yield of 1,2-dehydrotanshinone IIA sodium sulfonate is 24.8%, and the purity is 95.07%; the yield of tanshinone IIB sodium sulfonate The yield was 17.46%, and the purity was 96.14%; the yield of sodium 1-hydroxytanshinone IIA sulfonate was 29.45%, and the purity was 97.57%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com