Near-infrared fluorescent nanoparticle in-vivo probe and preparation method thereof
A near-infrared and internal fluorescence technology, applied in the field of preparation of near-infrared in vivo fluorescent nanoparticle probes and near-infrared in vivo fluorescent nanoparticle probes, can solve the problem of reducing the fluorescence intensity of quantum dots in vivo, harsh reaction conditions, inapplicability And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Step 1: Preparation of water-soluble near-infrared CdHgTe fluorescent quantum dots
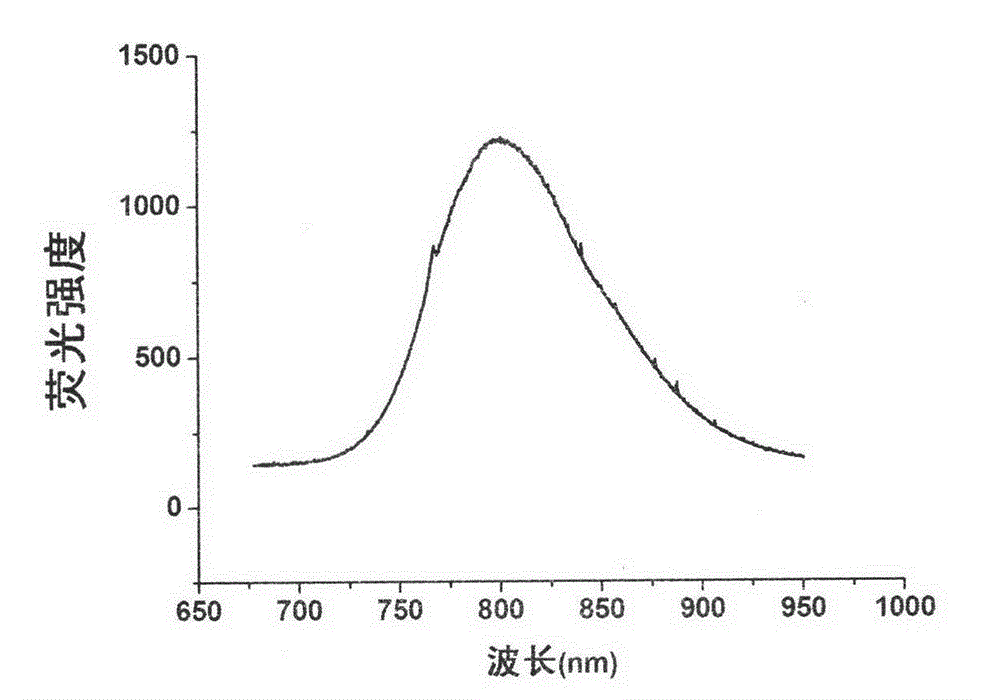
[0034] Accurately weigh 60mg of tellurium powder, NaBH 4 40mg, placed in a 25ml three-necked bottle, under N 2 Under air protection, 2 ml of double distilled water was added, and reacted in a water bath at 40° C. for 30 minutes to obtain a purple-red NaHTe solution. N was introduced into the solution 2 The solution was deoxygenated by air for 30 min. Another preparation 100ml containing 92.4mgCd(NO 3 ) 2 solution, and added 63 μl of mercaptoacetic acid (MPA) as a stabilizer, adjusted the pH to 11.2 with 1mol / L NaOH, and added 10 μl of mercuric nitrate solution. N was introduced into the solution 2 The solution was deoxygenated by air for 30 min. Under vigorous stirring, inject 600 μl of the above-mentioned deoxygenated 0.5mol / L NaHTe solution to make Cd 2+ : NaHTe: The molar ratio of MPA is 1: 0.5: 2.4, then rapidly heated to reflux to boiling. Reflux for 1 hour to obtain a CdH...
Embodiment 2
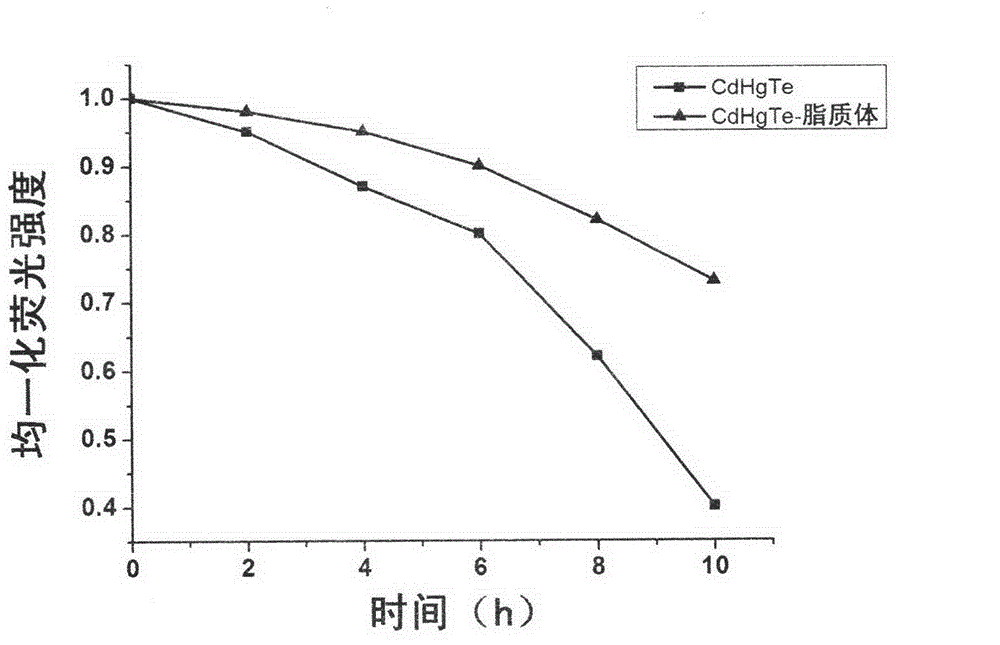
[0040] (1) Stability investigation of CdHgTe quantum dots and CdHgTe quantum dots-liposome fluorescent probes:
[0041]The CdHgTe quantum dots prepared in Example 1 and the CdHgTe quantum dot-liposome fluorescent probes were all placed under 20W254nm ultraviolet lamps for irradiation, and the fluorescence intensity was measured at 0, 2, 4, 6, 8, and 10 hours, and compared the two resistance to photobleaching. see results image 3 . It can be seen that the photostability of the CdHgTe quantum dot-liposome fluorescent probe is obviously better than that of the single CdHgTe quantum dot.
[0042] (2) Toxicity investigation of CdHgTe quantum dots and CdHgTe quantum dots-liposome fluorescent probes:
[0043] Select human breast cancer cell MCF-7 cells, add them into 96-well culture plate, set up five duplicate wells, set at 37°C, 5% CO 2 Cultivate overnight in the incubator, replace the culture medium with 0.5% serum M199 culture medium to synchronize the cells, then add the Cd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com