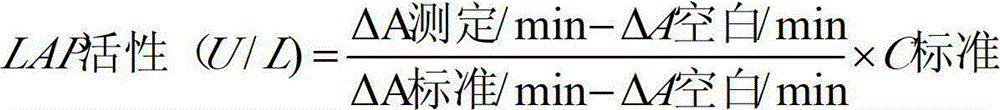Anti-heparin interference leucine aminopeptidase measuring reagent
A technology of leucine aminopeptidase and anti-interference agent, which is applied in the field of medical testing, can solve the problems of not being able to meet the clinical requirements for rapid return of test results, the time required for serum separation, and the false increase of test results, etc., to achieve good anti-interference. The effect of heparin interference performance, meeting the clinical test requirements, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
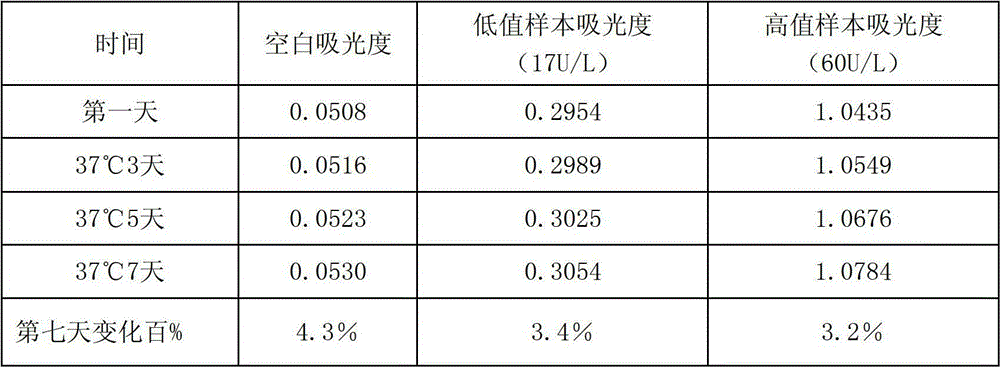
Embodiment 1
[0023] Reagent 1:
[0024] Tris hydroxymethyl amino buffer (pH 7.0) 50mmol / L
[0025] Tween 20 5ml / L
[0027] Reagent 2:
[0028] 2-Morpholineethanesulfonic acid buffer (pH 4.0) 100mmol / L
[0029] L-leucyl-p-nitroanilide 2mmol / L
[0030] Sucrose 5g / L
[0032] Reagent 1 and Reagent 2 can be in dry powder state and used after adding water to dissolve before use; they can also be made into liquid reagents and used directly;
Embodiment 2
[0034] Reagent 1:
[0035] N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid buffer (pH 8.0) 100mmol / L
[0036] Span-80 10ml / L
[0037] Potassium sorbate 1g / L;
[0038] Reagent 2:
[0039] Acetic acid-sodium acetate buffer (pH 3.0) 100mmol / L
[0040] L-leucyl-p-nitroanilide 5mmol / L
[0041] BSA 2g / L
[0043] Reagent 1 and Reagent 2 can be in dry powder state and used after adding water to dissolve before use; they can also be made into liquid reagents and used directly;
Embodiment 3
[0045] Reagent 1:
[0046] N-(2-hydroxyethyl)piperazine-N'-4-butanesulfonic acid buffer (pH 8.5) 500mmol / L
[0047] Triton X-100 20ml / L
[0048] Proclin300 1g / L;
[0049] Reagent 2:
[0050] Imidazole buffer (pH 6.0) 100mmol / L
[0051] L-leucyl-p-nitroanilide 20mmol / L
[0052] Sorbitol 5g / L
[0053] Proclin300 0.1g / L.
[0054] Reagent 1 and Reagent 2 can be in dry powder state and used after adding water to dissolve before use; they can also be made into liquid reagents and used directly;
[0055] The testing conditions for the kit of the present invention to measure LAP in samples are as follows: temperature: 30-37° C.; light path of cuvette: 1.0 cm. The main detection wavelength is 405nm.
[0056] The method of using the LAP determination kit of the present invention to measure LAP in the sample is as follows: add R1 to the sample (calibration tube as the sample) and mix evenly, after incubating at 30-37°C for 3-5min, immediately add R2 to mix evenly, and then add R2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com