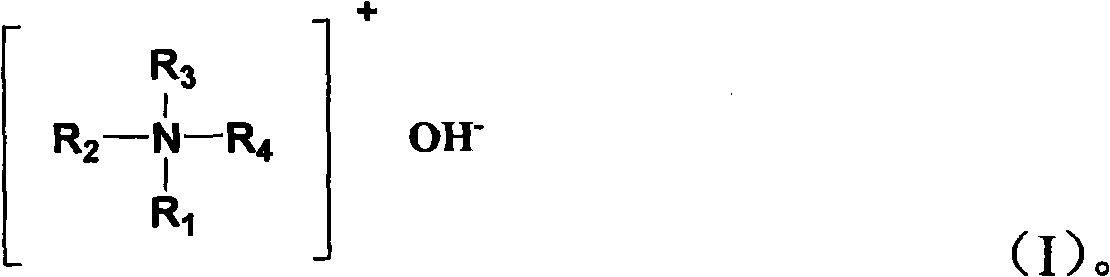Method for preparing cyclohexanone
A technology of cyclohexanone and cyclohexane, which is applied in the field of preparation of cyclohexanone, can solve the problems of low efficiency and low efficiency, and achieve the effects of environmental friendliness, good stability and high conversion rate of cyclohexane
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] This example is used to illustrate the method for preparing cyclohexanone according to the present invention.
[0074] 10 grams of TS-1 titanium-silicon molecular sieve (in terms of oxides, the titanium content in the titanium-silicon molecular sieve is 2.5% by weight), 4.8 grams of concentration is 28% by weight of tetraethylammonium hydroxide aqueous solution placed in the lining In the autoclave of polytetrafluoroethylene, and add 2mL of deionized water to the above autoclave, then close the autoclave, raise the temperature in the autoclave to 145°C, and react at this temperature for 12 hours . The reaction product was dried at 100° C. for 180 minutes, and then the dried product was calcined at 650° C. in an air atmosphere for 3 hours, thereby obtaining Catalyst A.
[0075] Put cyclohexane, hydrogen peroxide, acetone and catalyst A in an autoclave, and react for 12 hours at a temperature of 30° C. and a pressure of 1.5 MPa. Wherein, the molar ratio of cyclohexane, ...
Embodiment 2
[0085] This example is used to illustrate the method for preparing cyclohexanone according to the present invention.
[0086] 12 grams of TS-1 titanium-silicon molecular sieves (calculated as oxides, the titanium content in the titanium-silicon molecular sieves is 1.2% by weight), 1 gram of triethanolamine and 5 grams of tetra-n-propylammonium hydroxide with a concentration of 16.3% by mass The aqueous solution is placed in an autoclave lined with polytetrafluoroethylene, and 3 mL of deionized water is added to the autoclave, then the autoclave is closed, the temperature in the autoclave is raised to 160 ° C, and the The reaction was carried out at this temperature for 56 hours. The reaction product was dried at 150° C. for 120 minutes, and then the dried product was calcined at 540° C. in an air atmosphere for 6 hours, thereby obtaining Catalyst B.
[0087] Put cyclohexane, hydrogen peroxide, tert-butanol and catalyst B in an autoclave, and react for 12 hours at a temperatur...
Embodiment 3
[0089] This example is used to illustrate the method for preparing cyclohexanone according to the present invention.
[0090] 12 grams of TS-1 titanium-silicon molecular sieve (in terms of oxides, the titanium content in the titanium-silicon molecular sieve is 3.2% by weight), 0.5 gram of n-propylamine and 7.5 grams of tetra-n-propylammonium hydroxide with a concentration of 16.3% by mass The aqueous solution was placed in an autoclave lined with polytetrafluoroethylene, then the autoclave was closed, the temperature in the autoclave was raised to 175° C., and the reaction was carried out at this temperature for 48 hours. The reaction product was dried at 90° C. for 240 minutes, and then the dried product was calcined at 700° C. in an air atmosphere for 2 hours, thereby obtaining Catalyst C.
[0091] Put cyclohexane, hydrogen peroxide, methanol and catalyst C in an autoclave, and react for 12 hours at a temperature of 50° C. and a pressure of 0.5 MPa. Wherein, the molar ratio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com