Cell-based biological drug for allogeneic adoptive cellular immunotherapy for hematological diseases and preparation method
A technology of cellular immunity and blood system, which is applied in the field of cell-based biomedicine and preparation of allogeneic adoptive cellular immunotherapy for blood system diseases, can solve the problems of high mortality, poor quality of life of patients, and few applications, and achieve hematopoiesis Improvement of function, good tolerance of patients, and recovery of physical strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
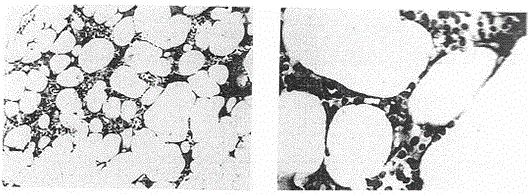
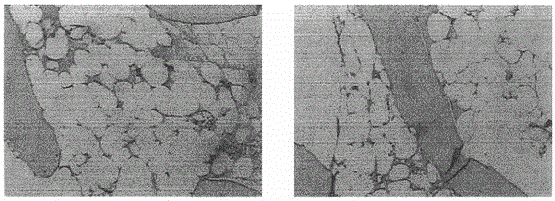
[0084] The invention provides a medicine for treating MDS and a preparation method thereof, the preparation steps comprising:
[0085] Step 1, co-cultivating immune cells and antigens in a liquid cell culture medium in a culture container, so as to obtain specific immune cell cultures with anti-corresponding antigens;
[0086] Step 2, isolating cellular biopharmaceuticals with an immune response from the culture obtained in step 1;
[0087] Wherein, in addition to immune cells, the liquid cell culture medium also includes components a)~e):
[0088] a) Cell mitogens: any one or more of concanavalin, phytohemagglutinin, pokeweed, lipopolysaccharide, dextran, and anti-CD3 antibody.
[0089] b) Antigen: The antigen is an abnormal clone of hematopoietic stem cells, and can be an antigen derived from allogeneic, heterogeneous, or self.
[0090] c) Exogenous superantigens, and / or endogenous superantigens: such as bacterial endotoxin, endogenous superantigens: such as any one or sev...
Embodiment 1
[0105] Allogeneic mononuclear cells are co-cultured with specific antigens (abnormal clones of hematopoietic stem cells) in a liquid medium, which also includes concanavalin, exogenous superantigens, lymphokines, and immune adjuvants, among which , the amount of concanavalin in the medium is 1 μg / L; the amount of exogenous superantigen (Pseudomonas aeruginosa toxin) in the medium is 5 μg / L; the amount of lymphokine (interleukin II) in the medium is 1000u / L; the dosage of immune adjuvant (5% Tween-80) was 0.1mL / L.
[0106] The ratio of T lymphocytes to specific antigen (Raji (Burkitt's lymphoma cells)) is 5:1 (cell number ratio).
[0107] The T lymphocytes are co-cultured with specific antigens for 30 days to obtain specific immune cell cultures with anti-corresponding antigens.
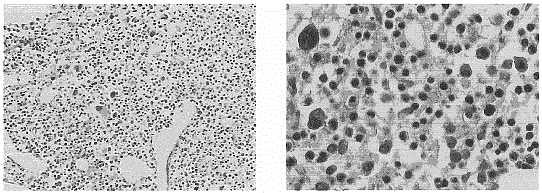
[0108] Cells with an immune response, mainly cytotoxic T lymphocytes, are isolated from the culture.
Embodiment 2
[0110] Allogeneic mononuclear cells (genetically modified T lymphocytes) are co-cultured with specific antigens (abnormal clones of hematopoietic stem cells) in a liquid medium that also includes phytohemagglutinin, endogenous superantigens , lymphokine, immune adjuvant, among which, the dosage of phytohemagglutinin (PHA) in the culture medium is 5 μg / L; the dosage of endogenous superantigen (Pseudomonas aeruginosa toxin) in the culture medium is 1 μg / L; (Interferon) in the culture medium at a dosage of 1000u / L; immune adjuvant (5% Tween-80) at a dosage of 0.1mL / L.
[0111] The ratio of T lymphocytes to specific antigen (MOLT-4 (acute lymphoblastic leukemia cells)) is 50:1 (cell number ratio).
[0112] Co-cultivate T lymphocytes with specific antigens for 90 days to obtain specific immune cell cultures against corresponding antigens.
[0113] Cells with an immune response, mainly cytotoxic T lymphocytes, are isolated from the culture.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com