Transmembrane peptide-mediated antisense antibacterial agent and preparation method and application thereof
A technology of membrane-penetrating peptide and antibacterial agent, which is applied in the field of antisense antibacterial agent and its preparation, and can solve the problems of difficult penetration and low intracellular concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 designs, synthesizes and screens the LNA of anti-Staphylococcus aureus ftsZ mRNA
[0031] (1) Screen the effective targets of ftsZ mRNA and design specific and efficient LNA: Use RNA Structure4.5 software to analyze the sequence of ftsZ mRNA in detail and predict its secondary structure. The basic length of LNA is 18-21 bases. Conserved sequence regions and non-folding regions select effective targets, and screen out the best 10 LNAs (see Table 1).
[0032] Table 1 Locked nucleic acid (LNA) designed for ftsZ mRNA
[0033]
[0034] Note: The uppercase letters in the nucleic acid sequence column represent LNA, and the lowercase letters represent DNA.
Embodiment 2
[0035] Preparation and separation and purification of embodiment 2 PLNA
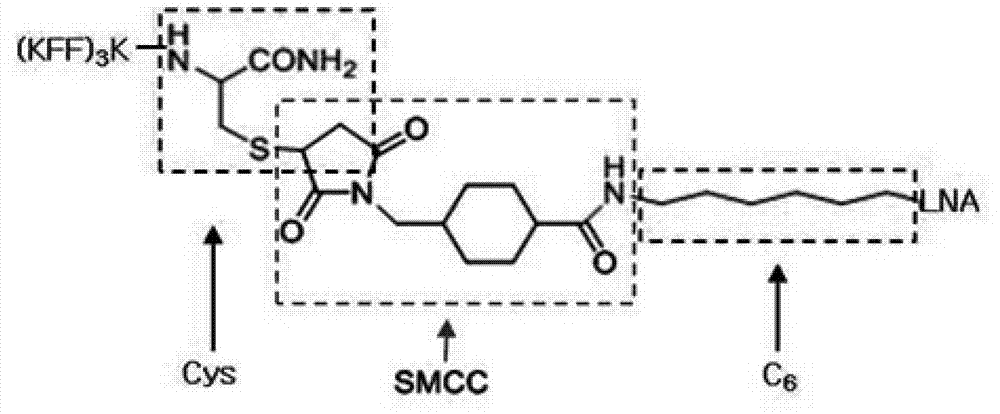
[0036] (1) Preparation of membrane-permeable peptide (KFF) 3K: synthesized by fluorenylmethoxycarbonyl solid-phase synthesis method (Fmoc-SPPS), that is, using HATU / DIEA as a condensation agent, on MBHA-Rink resin, using Fmoc to protect α- Amino acids, from the C-terminal (carboxyl-terminal) to the N-terminal (amino-terminal) solid-phase synthesis of polypeptides with cysteine at the C-terminal, the synthesized polypeptides are cleaved by high-concentration TFA and separated and purified by RP-HPLC. The amino acid sequence of the permeable peptide (KFF) 3K is KFFKFFKFFK, K is lysine (Lys), F is phenylalanine (Phe).
[0037] The specific steps are: the first step, solid-phase synthesis of polypeptides: the prepared polypeptides are carried out one by one from the C-terminus to the N-terminus. First, MBHA-Rink resin is packed into a column, and dichloromethane (CHCl 2 ), make it swell, deprotect with 20...
Embodiment 3
[0044] Embodiment 3 PLNA studies on the minimum inhibitory concentration of Mu50
[0045] According to the method of Example 2, it is combined with the permeable peptide (KFF) which promotes LNA to pass through the cell wall at the carbon end of LNA respectively. 3 K phase connection, prepared into PLNA.
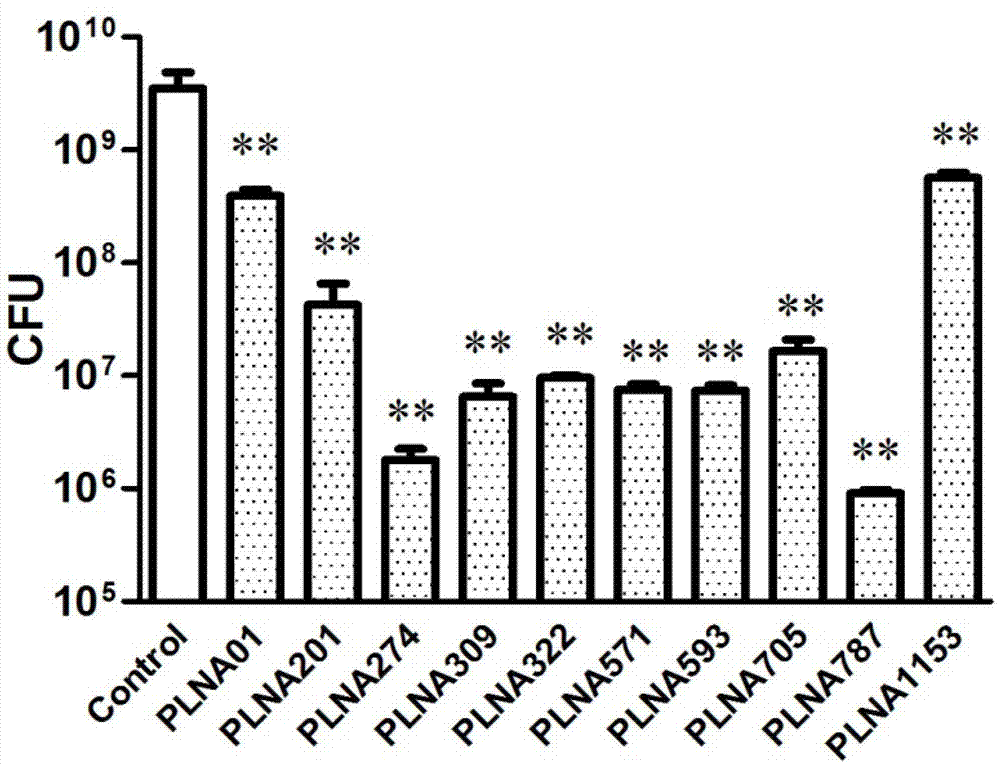
[0046] Combining improved microplate MIC determination method and colony counting method as a method for effective antibacterial antisense target screening. The inventor first determined the MIC of 10 anti-ftsZ PLNA sequences (anti-ftsZ PLNA 01, 201, 274, 309, 322, 571, 593, 705, 787 and 1153) to S. aureus (see Table 2), and the results It showed that all 10 PLNAs had different antibacterial activities. Each 10 μM PLNA was co-incubated with Mu50 for 8 hours, and the plate cloning experiment was carried out, and the CFU was counted. The results showed that 10 PLNAs could significantly reduce the number of Mu50 colonies ( figure 2 ). Based on the experimental results of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com