Method for preparing pesticide nitenpyram
A technology of nitenpyram and insecticide, which is applied in the field of preparation of nitenpyram, can solve the problems of low yield, unsafe operation, complicated preparation process, etc., and achieve industrial production, reduce environmental pollution, and produce The effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
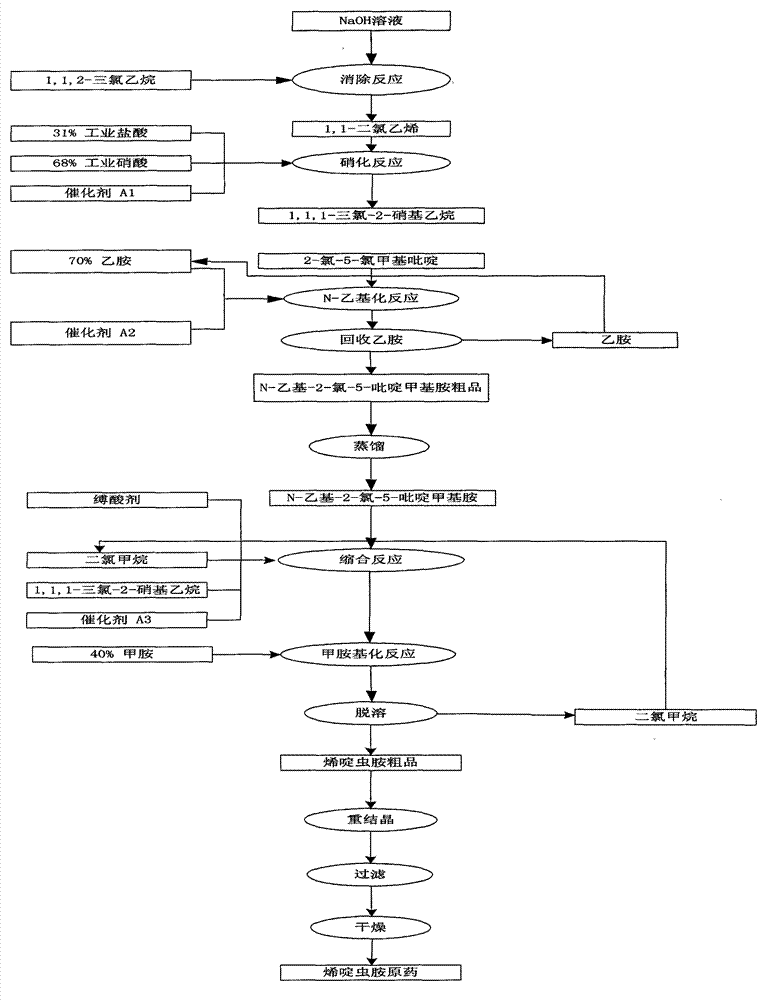
[0030] The present invention will be described in further detail below in conjunction with the accompanying drawings and embodiments.
[0031] Such as figure 1 Shown, present embodiment is carried out by following synthesis process route and step:
[0032] (1) elimination reaction, its reaction equation is:,
[0033]
[0034] The elimination reaction is carried out according to the following steps: in a 2000L enamel reaction kettle, add 450Kg1,1,2-trichloroethane, heat to a certain temperature, and evenly add 30% sodium hydroxide solution dropwise under vigorous stirring 450Kg, the dropping time is about 5 hours. During the reaction, the product vinylidene chloride escapes from the top of the distillation column, the temperature of the column top is 32-40°C, and enters the receiving tank after being condensed by the condenser tube. After dropping, continue the insulation reaction until substantially no product escapes. Then slowly raise the temperature to a certain temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com