Method for preparing ceftiofur hydrochloride suspension injection
The technology of ceftiofur hydrochloride and suspension injection is applied in the preparation of ceftiofur hydrochloride preparation and the field of preparation of ceftiofur hydrochloride suspension injection, which can solve the problems of low dissolution rate, low bioavailability and the like, and achieve preparation of ceftiofur hydrochloride. Stable performance, simple preparation process and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
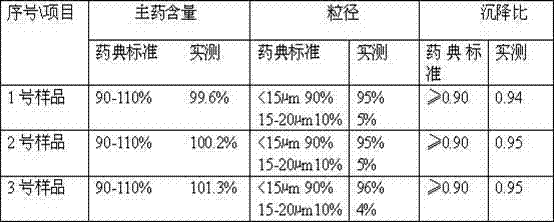
[0018] Weigh 5.4 g of ceftiofur hydrochloride, place it in a pulverizer, and pulverize until more than 90% of the particle size is ≤20 μm; take 100 mL of injection-grade soybean oil, heat it to 140 ° C in a container, stir and sterilize it for 60 minutes, and cool it to room temperature; Add the pulverized ceftiofur hydrochloride to the soybean oil, and stir to disperse evenly; slowly add sorbitan fatty acid ester under stirring, stir evenly, stir evenly, continue stirring evenly, and completely disperse into a suspension; The obtained suspension is ultra-fine through a homogenizer, and the specific operation is as follows: put the suspension of ceftiofur hydrochloride into four stainless steel containers in a high-performance ball mill, start the ball mill for 5 to 10 minutes, and grind the suspension into fine particles , making more than 90% of the particles ≤ 15 μm, the ceftiofur hydrochloride suspension injection is obtained.
[0019] The obtained ceftiofur hydrochloride ...
Embodiment 2
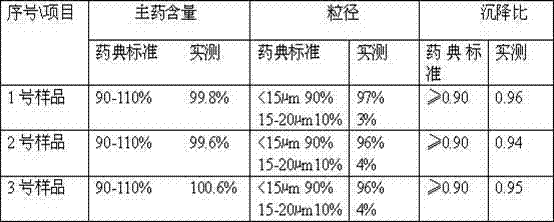
[0023] Weigh 5.6 g of ceftiofur hydrochloride, place it in a pulverizer, and pulverize until more than 90% of the particle size is ≤20 μm; take 100 mL of injection-grade soybean oil, heat it to 145 ° C in a container, stir and sterilize it for 55 minutes, and cool it to room temperature; Add the pulverized ceftiofur hydrochloride to the soybean oil, and stir to disperse evenly; slowly add sorbitan fatty acid ester under stirring, stir evenly, stir evenly, continue stirring evenly, and completely disperse into a suspension; Put the ceftiofur hydrochloride suspension into four stainless steel containers in a high-performance ball mill, start the ball mill for 5 to 10 minutes, and grind the suspension so that more than 90% of the particles are ≤15 μm to obtain the ceftiofur hydrochloride Furan suspension injection.
[0024] The obtained ceftiofur hydrochloride suspension injection was tested according to the 2006 edition of the imported veterinary drug quality standard and the ap...
Embodiment 3
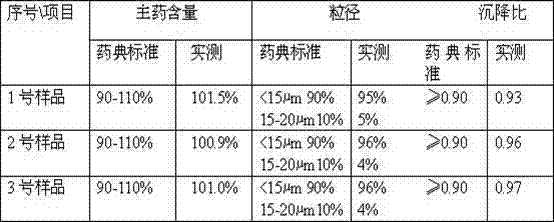
[0028] Weigh 5.7 g of cefotaxime hydrochloride, put it into a pulverizer and pulverize until more than 90% of the particles have a particle size of ≤20 μm; take 100 mL of injection-grade soybean oil and heat it to 150 ° C in a container to sterilize it for 50 minutes, and cool it to room temperature; Add the pulverized ceftiofur hydrochloride to the soybean oil, and stir to disperse evenly; slowly add sorbitan fatty acid ester under stirring, stir evenly, stir evenly, continue stirring evenly, and completely disperse into a suspension; Put the ceftiofur hydrochloride suspension into four stainless steel containers in a high-performance ball mill, start the ball mill for 5-10 minutes, and grind the suspension so that more than 90% of the particles are ≤15 μm, to obtain the cephalosporin hydrochloride Thifur Suspension Injection.
[0029] The obtained ceftiofur hydrochloride suspension injection was tested according to the 2006 edition of the imported veterinary drug quality sta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com