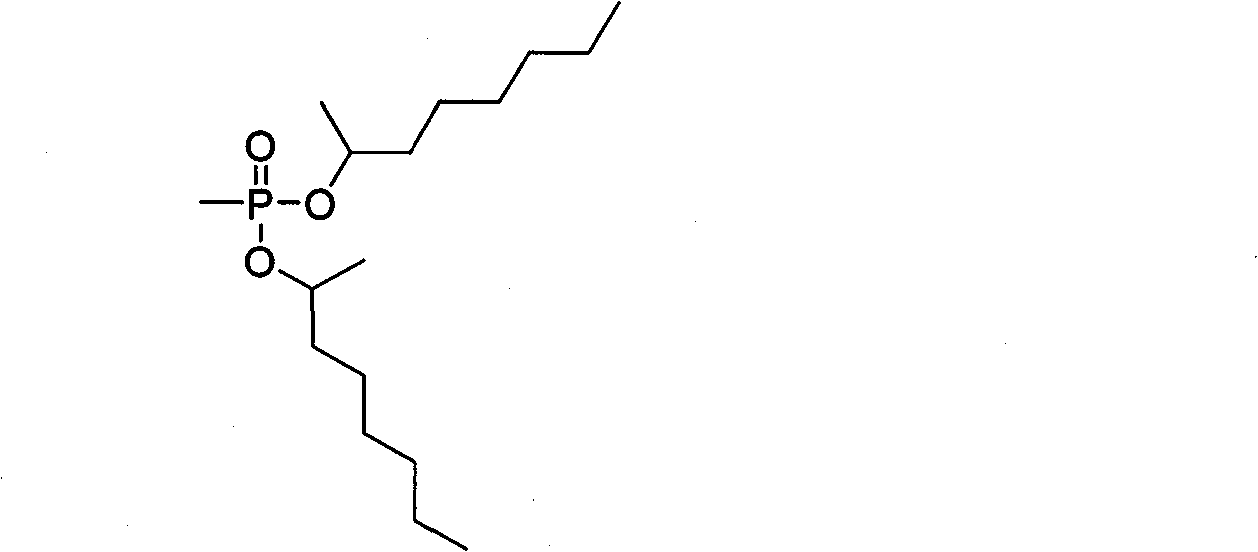
Synthesis method of dimethylheptyl methylphosphonate
A technology of dimethyl heptyl methyl phosphonate and dimethyl methyl phosphonate, which is applied in the field of preparation of organophosphorus compounds, can solve problems such as difficulty in raw material measurement, achieve low equipment investment, high yield, and reduce equipment investment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The synthetic method of methylheptyl phosphonate of the present invention may further comprise the steps:
[0024] A, the acyl chloride reagent is joined in the reaction vessel, and the acyl chloride reagent is a kind of in thionyl chloride, phosphorus pentachloride, triphosgene, and dimethyl methyl phosphonate (DMMP) is added dropwise to at room temperature In the acid chloride reagent, taking mol as the unit of measurement, the consumption of the acid chloride reagent is 2 times or 2.5 times or 3 times or 3.5 times or 4 times or 4.5 times or 5 times that of dimethyl methylphosphonate (DMMP), and Add a catalytic amount of catalyst, the catalyst is N, N-disubstituted formamide or N-containing aromatic heterocycle or N-substituted N-containing aromatic heterocycle or tertiary amine, stir evenly at room temperature, and then raise the temperature of the material in the reaction vessel to 60 ℃ or 150℃, keep warm for 1.5h or 2.5h or 2.5h, then distill under reduced pressure...
Embodiment 2
[0029] The synthetic method of methylheptyl phosphonate of the present invention may further comprise the steps:
[0030] (1) Acyl chloride reaction: Add a mixture of 124.08 g (1 mol) of dimethyl methylphosphonate and 0.8 g of pyridine (0.01 mol) dropwise to 520.6 g of PCl at 20°C 5 (2.5mol), keep stirring at room temperature for 1h, heat up to 80°C, keep warm for 2h, and collect the substance with a boiling point of 56-57°C / 14mmHg by distillation under reduced pressure to obtain methylphosphonodichloride with a yield of 94.2% and a purity of 98.1%.
[0031] (2) Esterification reaction: under stirring, slowly add 14.9g of methylphosphonic dichloride (0.1mol) into 65.1g of secondary octanol (0.5mol), the generated HCl gas is absorbed with lye, and the generated Chloromethane collection. First react at room temperature (about 30°C) for 45min, then react at 55°C for 1.5h, and finally react at 85°C for 2h.
[0032] (3) Post-treatment: the reaction solution was cooled to room tem...
Embodiment 3
[0036] The synthetic method of methylheptyl phosphonate of the present invention may further comprise the steps:
[0037](1) Acid chlorination reaction: Slowly add a mixture of 124.08g dimethyl methylphosphonate (1mol) and 0.73gDMF (0.01mol) dropwise to 297.5g thionyl chloride (2.5mol), and heat to reflux, After 4 hours of heat preservation, the reaction solution was cooled, and the substance with a boiling point of 56-57° C. / 14 mmHg was collected by distillation under reduced pressure to obtain methylphosphonic dichloride with a yield of 96.4% and a purity of 98.5%.
[0038] (2) Esterification: under stirring, slowly add 14.9g of methylphosphonic dichloride (0.1mol) into 78.1g of 2-octanol (0.6mol), the generated HCl gas is absorbed with lye, and the generated Chloromethane collection. First react at room temperature (about 30°C) for 45min, then react at 55°C for 1.5h, and finally react at 85°C for 2h.
[0039] (3) Post-treatment: the reaction solution was cooled to room te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com