Dihalofluorescein derivative and application thereof
A fluorescein and derivative technology, applied in the field of fluorescent probes, can solve the problems of unreported non-labeled fluorescent probes, unfavorable selective detection, etc., achieve high selectivity and rapid response ability, prevent and detect cancer, high The effect of sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
[0023] Add 22.0g (160mmol) of p-nitrotoluene to 53.0mL of chlorosulfonic acid, and react at 60°C for 48h. After the reaction was completed, it was cooled to room temperature, then slowly poured into crushed ice, and extracted with 1 L of ether. The organic phase was washed with saturated brine (500mL×3), and after combining the organic phases, 300mL of concentrated ammonia water was added, and heated to 50°C until all the ether was volatilized. The reaction solution was cooled to room temperature, filtered, and the crude product was recrystallized twice from water to obtain 12.1 g of light yellow needle-like crystals with a yield of 35%, m.p.182-183°C.
[0024]
[0025] 9.0 g (900 mmol) of chromium trioxide was dissolved in a mixed solvent of 84.0 mL of concentrated sulfuric acid and 67.0 mL of deionized water, stirred in an ice bath until cooled to room temperature. Then, 4.3 g (20 mmol) of compound 1 was added to the reaction solution in batches, and stirred ...
Embodiment 2
[0055] Spectral performance test of compound shown in formula I
[0056] 1) Determination of ultraviolet and fluorescence spectra:
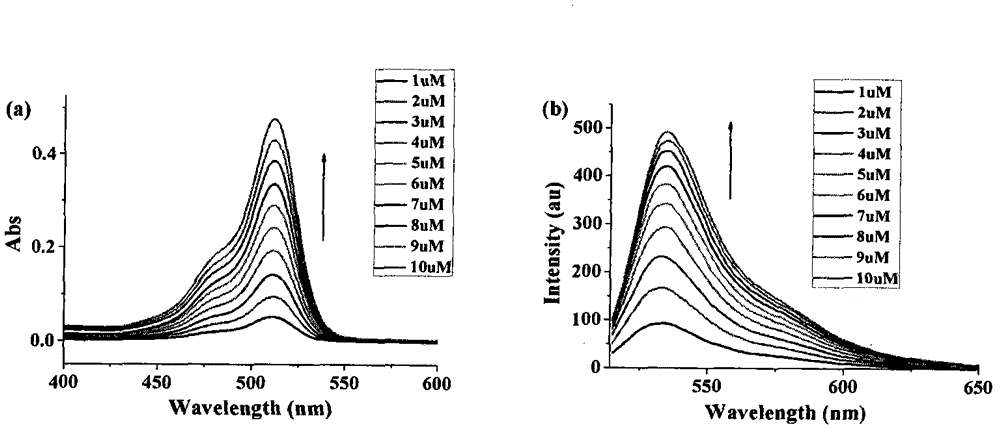
[0057] After the compound shown in formula I (hereinafter abbreviated as Z1) was vacuum-dried, the sample was accurately weighed on the balance (accurate to 0.0001 gram), fixed to volume with dimethyl sulfoxide, and formulated as 10 -3 The solution of M is fixed in a 10mL volumetric flask, and this is used as mother liquor to prepare different concentrations of Z1 solutions for the absorption spectrum and excitation-emission spectrum tests. The results are shown in Table 1 (Z1 is in Tris-HCl (containing 0.01% DMSO , 0.1 mM ZnCl 2 ) spectral data of the buffer):
[0058] Table 1
[0059]
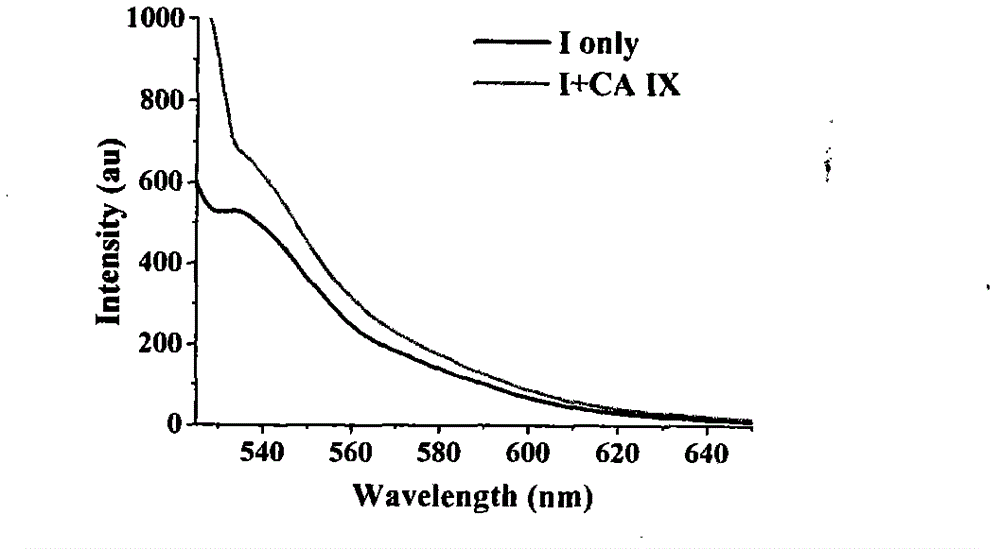
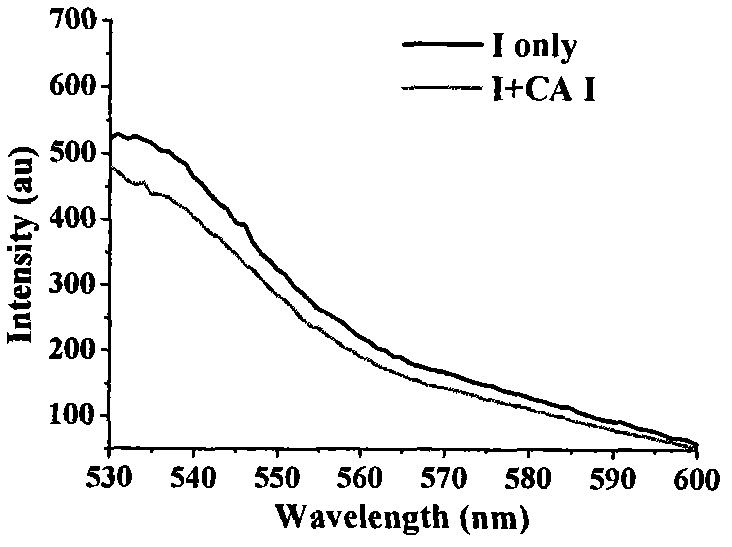
[0060] 2) The fluorescence responses of Z1 to carbonic anhydrase I, II and IX:
[0061] Will 1×10 -3The standard probe mother solution of mol / L is diluted to 0.1 μ M solution with Tris-HCl buffer solution (comprising 0.01% DMSO and 0.1 mM ZnCl 2 ), and ...
Embodiment 3
[0063] Determination of Z1 inhibition constants for carbonic anhydrase I, II and IX
[0064] We use the Stopped-flow method to determine the inhibition constant, the principle is to use carbonic anhydrase to catalyze CO 2 hydration, thereby changing the pH value of the test system, and then using the absorption of the detection phenol red indicator at 557nm at different pHs to calculate the inhibition constant of the probe and enzyme, specifically see Table 2 (Z1, AZA (control, purchased From Sigma Company) and EZA (control, purchased from Sigma Company) to carbonic anhydrase I, II and IX inhibition constants).
[0065] Table 2
[0066]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com