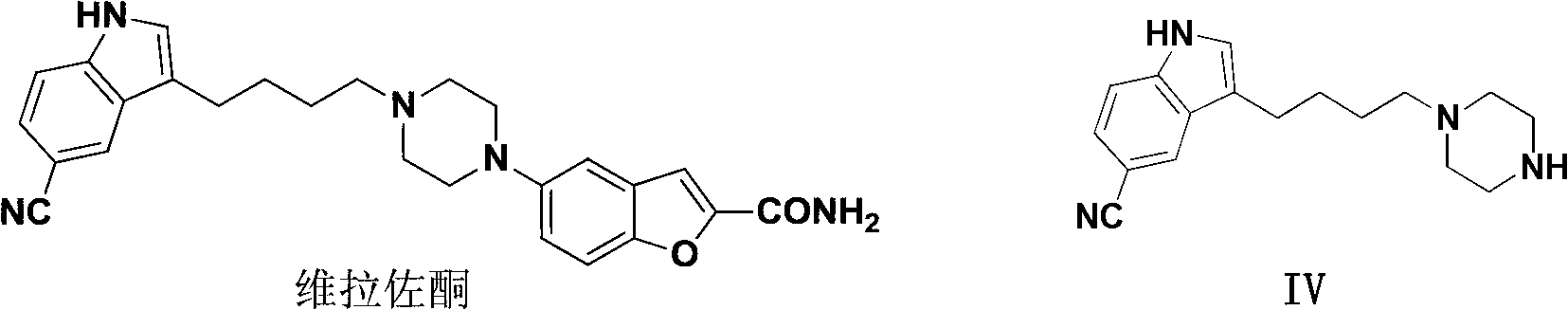
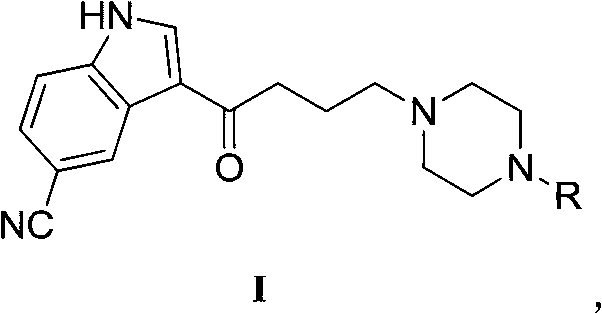
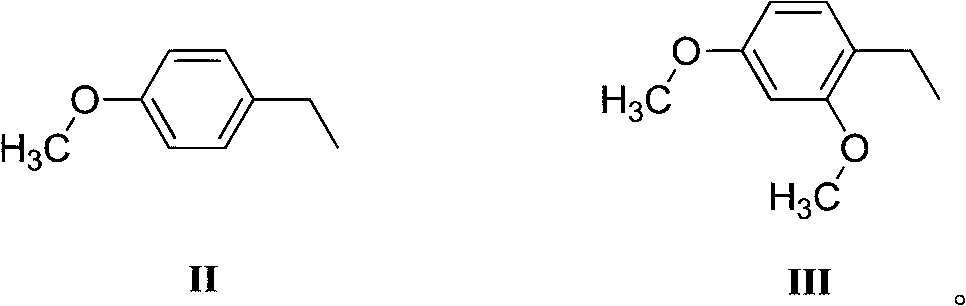3-(4-(4-substituted-piperazino)-1-butyryl)indolyl-5-formonitrile and application thereof
A piperazine and protecting group technology, applied in the field of medicinal chemistry, can solve problems such as unmentioned and increased synthesis costs, and achieve the effects of increased reactivity, low reaction cost, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0044]Preparation Example 1 Preparation of 3-(4-(4-(4-methoxybenzyl)piperazine)-1-butyryl)indole-5-carbonitrile via ethyl bromobutyrate
[0045] 1) Preparation of ethyl 4-(4-(4-methoxybenzyl)piperazine)-1-butyrate
[0046]
[0047] At 25°C, add 1-(4-methoxybenzyl)piperazine (500.0g, 2.42mol) and ethyl 4-bromobutyrate (520.1g, 2.67mol) into 5L of acetonitrile, stir until completely dissolved , and potassium carbonate (501.7 g, 3.63 mol) was added. Heat up to 80°C and keep stirring for about 6 hours. TLC showed that the reaction was complete, filtered, and the filtrate was concentrated under reduced pressure. To the concentrated residue were added 3 L of 1N hydrochloric acid and 1 L of ethyl acetate, mixed thoroughly, and the aqueous layer was separated and washed with 1 L of ethyl acetate. The aqueous phase was adjusted to pH 10 with 6N sodium hydroxide solution, extracted twice with 2 L of dichloromethane, the organic phases were combined, washed with 1 L of saturated br...
preparation example 2
[0058] Preparation Example 2 Preparation of 3-(4-(4-(4-methoxybenzyl)piperazine)-1-butyryl)indole-5-carbonitrile via tert-butyl bromobutyrate
[0059] 1) Preparation of tert-butyl 4-(4-(4-methoxybenzyl)piperazine)-1-butyrate
[0060]
[0061] At 25°C, add 1-(4-methoxybenzyl)piperazine (20.0g, 97.0mmol), tert-butyl 4-bromobutyrate (22.7g, 101.8mmol) into 200ml of acetonitrile, stir, and dissolve After clearing, potassium carbonate (20.1 g, 145.4 mmol) was added. Warming up to 70° C. and stirring for about 8 hours. TLC showed that the reaction was complete, filtered, and the filtrate was concentrated under reduced pressure. Add 200ml of 0.1N hydrochloric acid and 100ml of ethyl acetate to the concentrated residue, mix well, separate the water layer, and wash with 100ml of ethyl acetate. The aqueous phase was adjusted to pH 10 with 6N sodium hydroxide solution, extracted twice with 200 ml of ethyl acetate, the organic phases were combined, washed with 100 ml of saturated br...
preparation example 33
[0069] Preparation Example 3 Preparation of 3-(4-(4-(2,4-dimethoxybenzyl)piperazine)-1-butyryl)indole-5-carbonitrile
[0070] 1) Preparation of 4-(4-(2,4-dimethoxybenzyl)piperazine)-1-butyrate
[0071] Referring to the first step in Preparation Example 1 or 2, using 1-(4-(2,4-dimethoxybenzyl))piperazine as raw material, 4-(4-(2,4-di Methoxybenzyl)piperazine)-1-butyric acid ethyl ester and 4-(4-(2,4-dimethoxybenzyl)piperazine)-1-butyric acid tert-butyl ester.
[0072] 2) Preparation of 4-(4-(2,4-dimethoxybenzyl)piperazine)-1-butanoic acid
[0073] Referring to the second step in Preparation Example 1 or 2, 4-(4-(2,4-dimethoxybenzyl)piperazine)-1-butyric acid can be prepared.
[0074] 3) Preparation of 4-(4-(2,4-dimethoxybenzyl)piperazine)-1-butyryl chloride hydrochloride
[0075] With reference to the third step in Preparation Example 1 or 2, 4-(4-(2,4-dimethoxybenzyl)piperazine)-1-butanoic acid reacts with thionyl chloride to obtain 4- (4-(2,4-dimethoxybenzyl)piperazine)-1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com