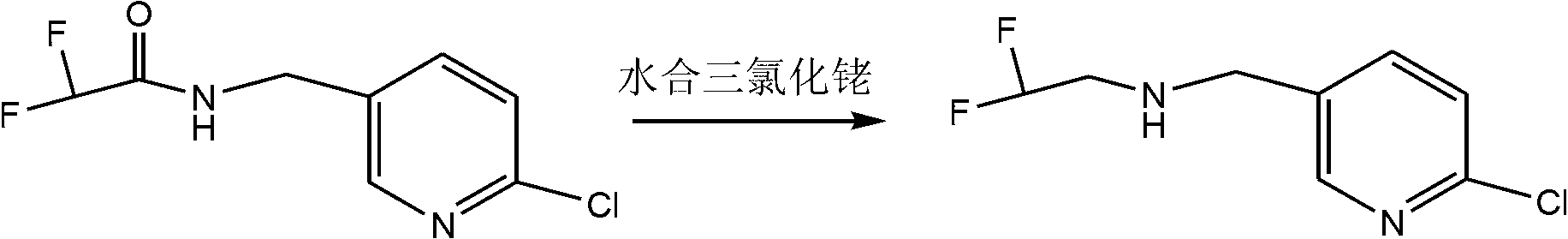
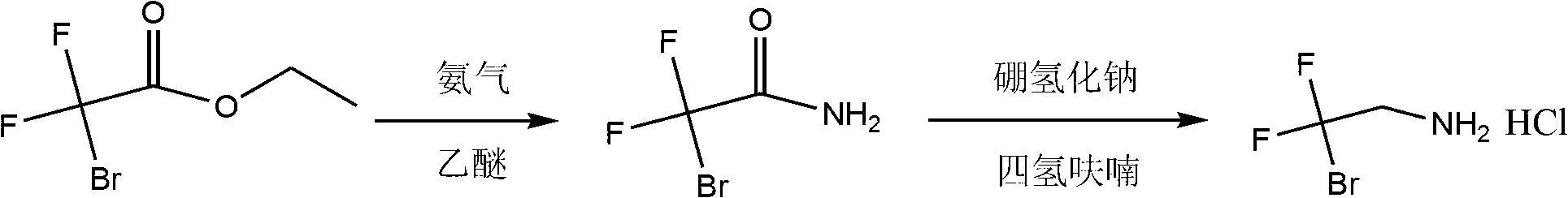
Synthesis method for 2-bromo-2,2-difluoroethylamine hydrochloride
A technology for difluoroethylamine hydrochloride and difluoroacetamide is applied in the field of synthesizing 2-bromo-2,2-difluoroethylamine hydrochloride, and the synthesis cost is reduced, the raw materials are cheap and easy to obtain, and the product quality is excellent. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] First step response:
[0020]
[0021] 700 g of ethyl 2-bromo-2,2-difluoroacetate was dissolved in 350 g of ether (the mass ratio of ethyl 2-bromo-2,2-difluoroacetate to ether was 2:1). Ammonia gas was continuously fed into the solution until the reaction of raw materials was detected by gas chromatography. The solvent was distilled off under reduced pressure to obtain a solid, which was washed with n-hexane and filtered. The filter cake was washed with n-hexane and dried under vacuum to obtain 600 g of off-white solid 2-bromo-2,2-difluoroacetamide, which was directly used in the next reaction without further purification.
[0022] Second step reaction:
[0023]
[0024] 600 grams of 2-bromo-2,2-difluoroacetamide was dissolved in 4.2 kg of tetrahydrofuran (the mass ratio of 2-bromo-2,2-difluoroacetamide and tetrahydrofuran was 1:7), and 350 grams of hydroboration was added Sodium (the molar ratio of 2-bromo-2,2-difluoroacetamide and sodium borohydride is 1:2.6)...
Embodiment 2
[0026] First step response:
[0027] 2 kg of ethyl 2-bromo-2,2-difluoroacetate was dissolved in 1.4 kg of ether (the mass ratio of ethyl 2-bromo-2,2-difluoroacetate to ether was 2:1.4). Ammonia gas was continuously fed into the solution until the reaction of raw materials was detected by gas chromatography. The solvent was distilled off under reduced pressure to obtain a solid, which was washed with n-hexane and filtered. The filter cake was washed with n-hexane and dried under vacuum to obtain 1.8 kg of off-white solid 2-bromo-2,2-difluoroacetamide, which was directly used in the next reaction without further purification.
[0028] Second step reaction:
[0029] 1.8 kg of 2-bromo-2,2-difluoroacetamide was dissolved in 13 kg of tetrahydrofuran (the mass ratio of 2-bromo-2,2-difluoroacetamide and tetrahydrofuran was 1:7.2), and 1 kg of hydroboration Sodium (the molar ratio of 2-bromo-2,2-difluoroacetamide and sodium borohydride is 1:2.5). Then control the reaction temperatu...
Embodiment 3
[0031] First step response:
[0032] 1 kg of ethyl 2-bromo-2,2-difluoroacetate was dissolved in 500 g of ether (the mass ratio of ethyl 2-bromo-2,2-difluoroacetate to ether was 2:1). Ammonia gas was continuously fed into the solution until the reaction of raw materials was detected by gas chromatography. The solvent was distilled off under reduced pressure to obtain a solid, which was washed with n-hexane and filtered. The filter cake was washed with n-hexane and dried under vacuum to obtain 880 g of off-white solid 2-bromo-2,2-difluoroacetamide, which was directly used in the next reaction without further purification.
[0033] Second step reaction:
[0034] 880 grams of 2-bromo-2,2-difluoroacetamide were dissolved in 5.3 kilograms of tetrahydrofuran (the mass ratio of 2-bromo-2,2-difluoroacetamide and tetrahydrofuran was 1:6), and 387 grams of hydroboration Sodium (the molar ratio of 2-bromo-2,2-difluoroacetamide and sodium borohydride is 1:2). Then control the reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com