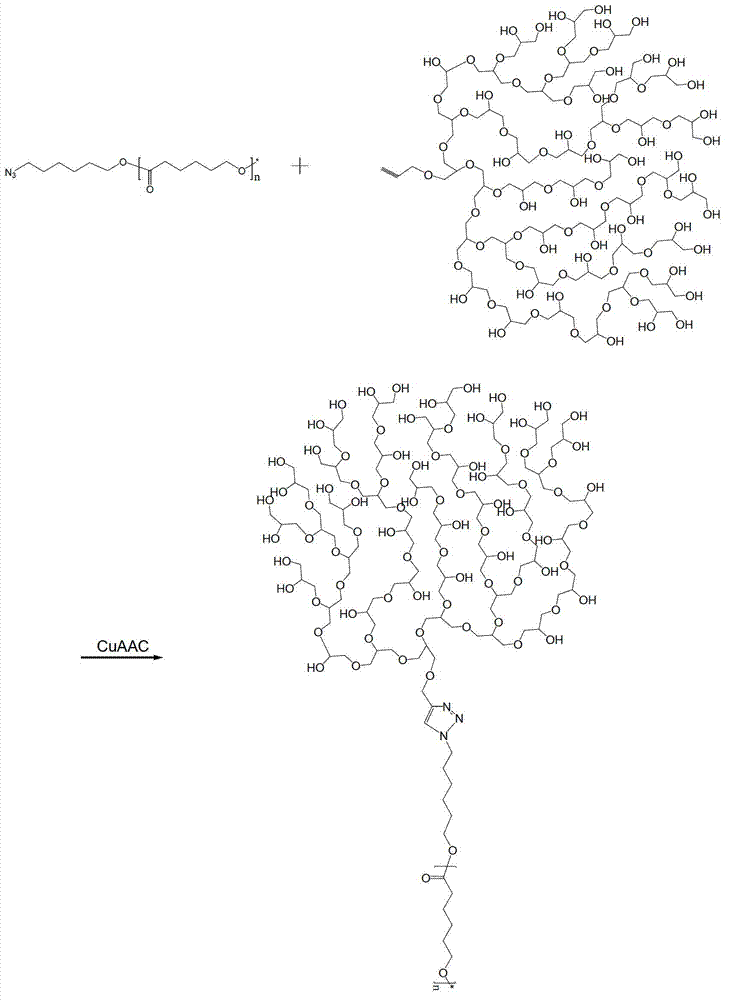
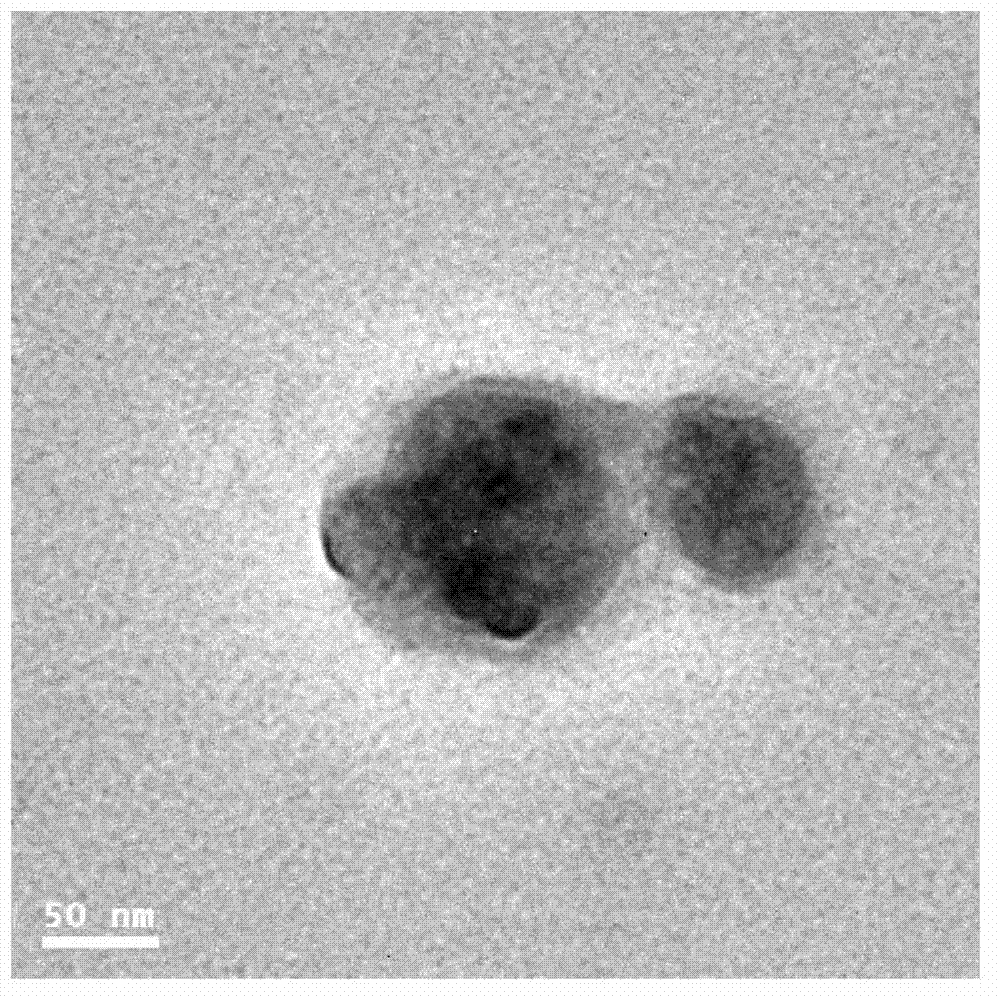
Preparation method and application of tree-like polyester-polyglycidol block polymer
A technology of polyglycidol and block copolymer, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, drug combinations, etc. It can solve the problems of loss of activity, reduction of post-modification functions, etc., and achieve Increased inhibition rate and inhibition of tumor angiogenesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A kind of preparation method of tree type polyester-polyglycidyl block copolymer, the steps are as follows:
[0033] 1) Add lactide in the polymerization sealed tube, then add 6-bromohexanol with a molar ratio of 1:30 to lactide to form the reactant, add stannous octoate as the catalyst, and the mass of the catalyst added is the reaction 0.1% of the amount of the substance, evacuated nitrogen replacement twice, sealed the tube at a vacuum of 5Pa, and reacted at a temperature of 110°C for 24 hours to obtain a polyester polymer with a halogenated end group;
[0034] 2) Under the protection of nitrogen, dissolve the above polymer and sodium azide with a molar ratio of 1:3 in DMF solvent to obtain a solution. The concentration of the polymer is 100g / L solution, react at room temperature for 24 hours, and then use acetic acid Ethyl extraction can obtain the azide-terminated polyester polymer;
[0035] 3) In the glycidyl ether monomer, add propynyl alcohol with a molar ratio...
Embodiment 2
[0042] A kind of preparation method of tree type polyester-polyglycidyl block copolymer, the steps are as follows:
[0043] 1) Add glycolide in the polymerization sealed tube, then add 6-bromohexanol with a molar ratio of 1:70 to glycolide to form the reactant, add stannous octoate as catalyst, and the amount of catalyst added is the reaction 0.1% of the substance mass, evacuated nitrogen replacement 3 times, sealed the tube at a vacuum degree of 40Pa, and reacted at a temperature of 135°C for 48 hours to obtain a polyester polymer with a halogenated end group;
[0044] 2) Under the protection of nitrogen, dissolve the above polymer and sodium azide with a molar ratio of 1:3 in DMF solvent to obtain a solution. The concentration of the polymer is 150g / L solution, react at room temperature for 24 hours, and then use acetic acid Ethyl extraction can obtain the azide-terminated polyester polymer;
[0045] 3) In the glycidyl ether monomer, add propynyl alcohol with a molar ratio ...
Embodiment 3
[0050] A kind of preparation method of tree type polyester-polyglycidyl block copolymer, the steps are as follows:
[0051] 1) Add caprolactone in the polymerization sealed tube, then add 6-bromohexanol with a molar ratio of 1:100 to caprolactone to form a reactant, add stannous octoate as a catalyst, and the amount of catalyst added is the reaction 0.1% of the substance mass, evacuated nitrogen replacement 4 times, sealed the tube at a vacuum degree of 80Pa, and reacted at a temperature of 150°C for 60 hours to obtain a polyester polymer with a halogenated end group;
[0052] 2) Under the protection of nitrogen, dissolve the above polymer and sodium azide with a molar ratio of 1:3 in DMF solvent to obtain a solution. The concentration of the polymer is 200g / L solution, react at room temperature for 24 hours, and then use acetic acid Ethyl extraction can obtain the azide-terminated polyester polymer;
[0053] 3) In the glycidyl ether monomer, add propynyl alcohol with a molar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com