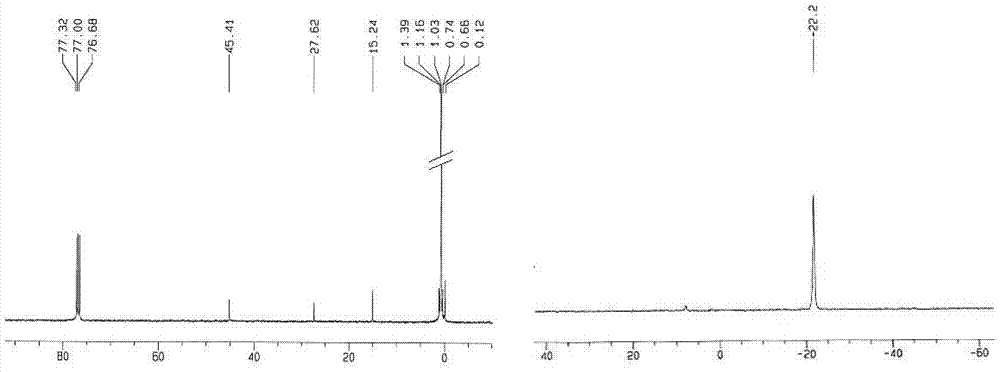
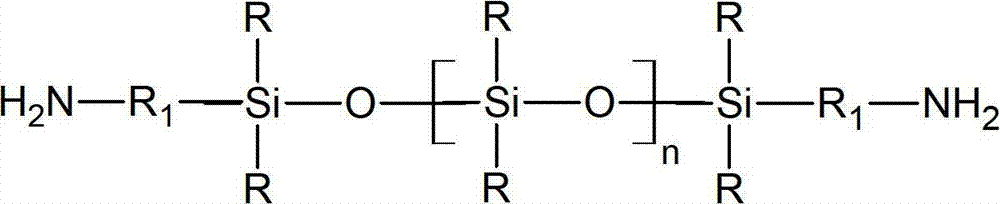
Preparation method for aminoalkyl ended polysiloxane
A technology of polyorganosiloxane and aminoalkyl sealing is applied in the field of preparation of polyorganosiloxane, which can solve problems such as low molecular weight and achieve the effects of improving flexibility, high and low temperature resistance, and improving adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Synthesis of N-trimethylsilylallylamine (Step 1)
[0039] In a 1L three-necked flask equipped with magnetic stirring, a reflux condenser, and a dropping funnel, after purging with nitrogen, add 195.5 g (1.8 mol) of trimethylchlorosilane, 363 g of triethylamine, and 100 ml of n-hexane in sequence, and heat under stirring. To 50°C, 85.8g (1.5mol) of allylamine was added dropwise within 1 hour, and after the addition, the temperature was raised to 70°C for 1 hour to react. The triethylamine hydrochloride produced by the reaction is removed by filtration, and the excess trimethylchlorosilane and solvent are distilled off from the filtrate, and then rectified with a 60cm fractionating column to collect a fraction at 108-110°C to obtain the product N-trimethylsilylene Propylamine 178.4g., yield 92%.
Embodiment 2
[0041]This embodiment is basically the same as Embodiment 1. The difference is that the feeding method of trimethylchlorosilane and allylamine is reversed, that is, allylamine is added to the flask, trimethylchlorosilane is added dropwise in 1 hour, and then reacted at 60°C for 2 hours, and the product N- Trimethylsilyl allylamine 157.1g, yield 81%.
Embodiment 3
[0043] This embodiment is basically the same as Embodiment 1. The difference is that the reaction does not add solvent n-hexane, but the HCl absorbent triethylamine is increased to 405g, and the reaction is carried out at 80°C for 1 hour, and the product N-trimethylsilylallylamine is 184.3g, and the yield is 95%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com