Preparation method of b-heterocyclic ketone compound
A technology of ketone compounds and compounds, which is applied in the field of preparation of β-heterocyclic substituted ketone compounds, can solve the problems of large industrial wastewater, complicated operation, high production cost, etc., and achieve high reaction yield, safe and convenient operation, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of 4-(2-5-methoxythiophene)-nonan-2-one: the molar ratio of feed material is 3-nonen-2-one: heterocyclic compound: catalyst is 1.0: 2.0: 0.1, wherein hetero The cyclic compound is 2-methoxythiophene, the catalyst is palladium dichloride, the organic solvent is tetrahydrofuran, and the dosage thereof is 10 times that of 3-nonen-2-one. Add 70.1 mg (0.5 mmol) of 3-nonen-2-one, 114.2 mg (1.0 mmol) of 2-methoxythiophene, and 8.8 mg (0.05 mmol) of palladium dichloride into a 25 mL Schlinker bottle equipped with a magnet. ), 1.0 mL of tetrahydrofuran, stirred at 0° C., and the reaction was complete after 0.25 h. After the reaction was complete, the solvent was removed under reduced pressure, and the product was separated and purified by silica gel column chromatography (petroleum ether: ethyl acetate=10:1) to obtain the 4-(2-5-methoxythiophene)-nonyl -2-Kone liquid, pure product 123.4mg, yield 97%.
Embodiment 2
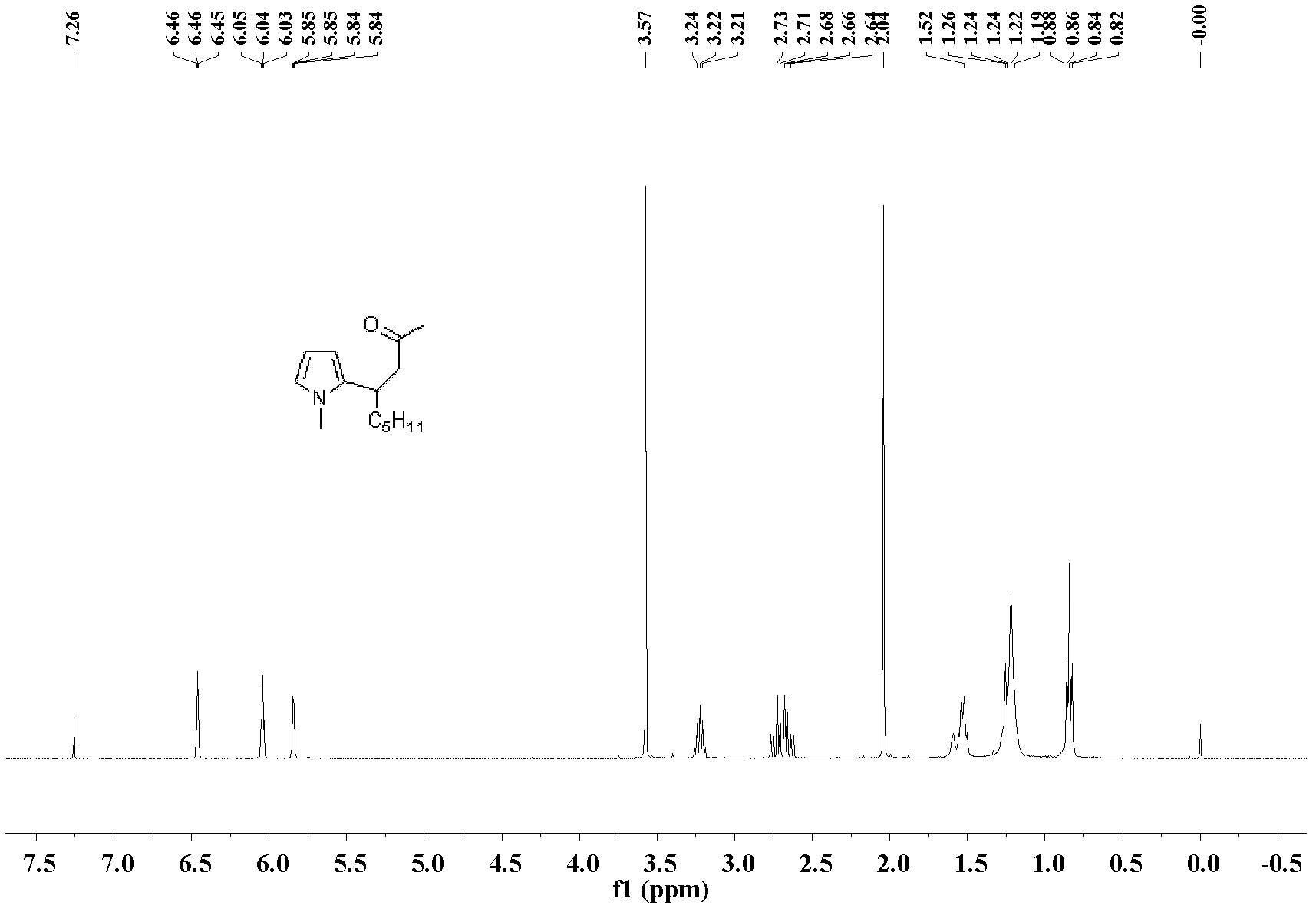
[0030] The preparation of 4-(2-5-methylfuran)-non-2-one: the molar ratio of feed material is 3-nonen-2-one: heterocyclic compound: catalyst is 1.0: 3.0: 0.1, wherein heterocyclic The compound is 2-methylfuran, the catalyst is palladium acetate, the organic solvent is n-hexane, and the dosage thereof is 60 times that of 3-nonen-2-one. Into a 25 mL Schlink bottle equipped with a magnet, add 70.1 mg (0.5 mmol) of 3-nonen-2-one, 123.1 mg (1.5 mmol) of 2-methylfuran, 11.2 mg (0.05 mmol) of palladium acetate, n-hexyl Add 5.0 mL of alkanes, stir the reaction at 60°C, and the reaction is complete after 10 hours. After the reaction was complete, the solvent was removed under reduced pressure, and the product was separated and purified by silica gel column chromatography (petroleum ether: ethyl acetate=10:1)) to obtain the 4-(2-5-methylfuran)-nonyl- 2-ketone liquid, pure product 108.9 mg, yield 98%.
Embodiment 3
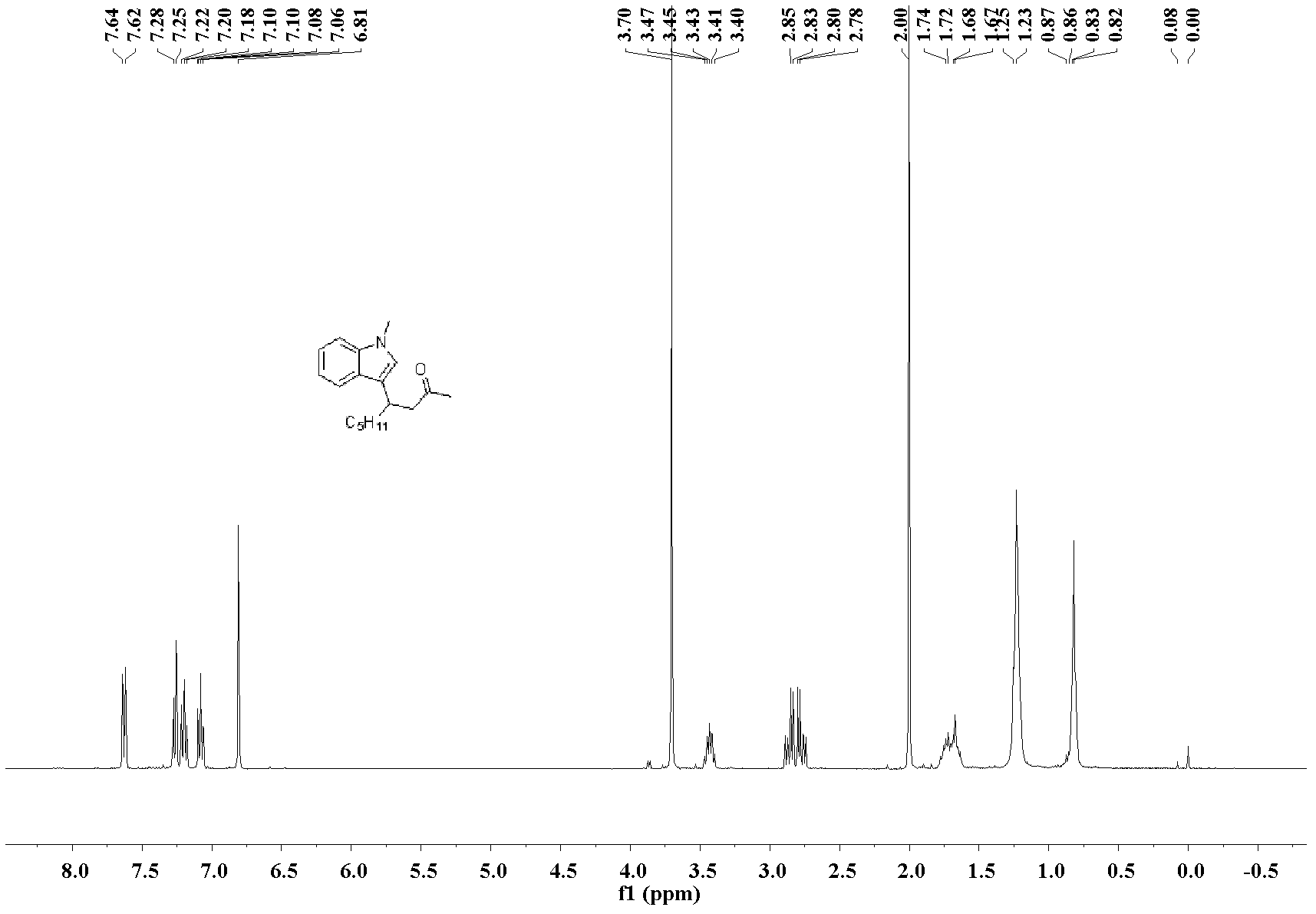
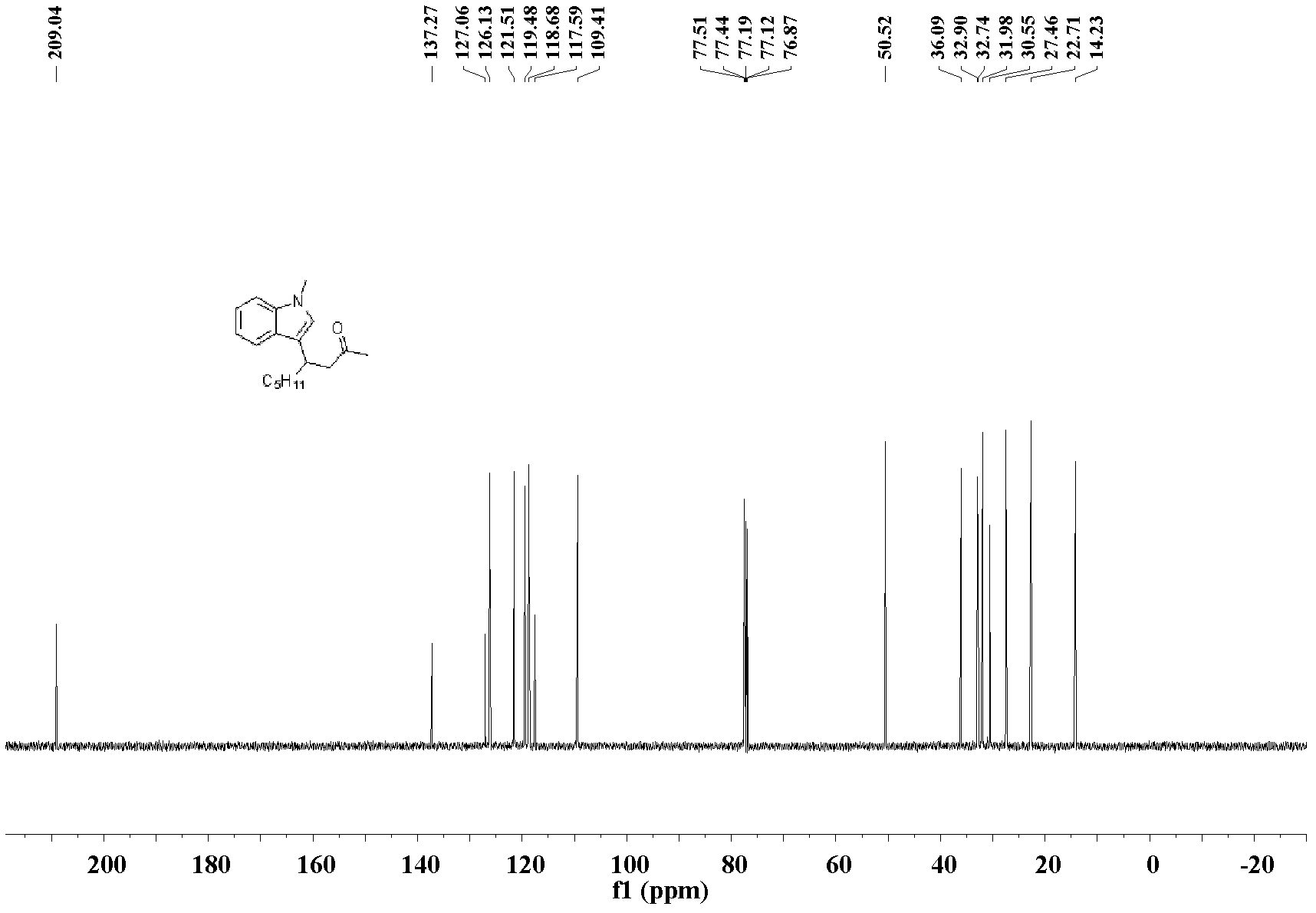
[0032] The preparation of 4-(3-N-methylindole)-non-2-one: the molar ratio of feed material is 3-nonen-2-one: heterocyclic compound: catalyst is 1.0: 1.0: 0.1, wherein hetero The cyclic compound is N-methylindole, the catalyst is bis(acetonitrile)palladium dichloride, the organic solvent is n-butanol, and its consumption is 100 times that of 3-nonen-2-one. In a 25mL Schlink bottle equipped with a magnet, add 3-nonen-2-one 70.1mg (0.5mmol), N-methylindole 65.6mg (0.5mmol), bis(acetonitrile)palladium dichloride 13.0 mg (0.05mmol), n-butanol 9.0mL, stirred and reacted at 130°C, and the reaction was complete after 48h. After the reaction was complete, the solvent was removed under reduced pressure, and the product was separated and purified by silica gel column chromatography (petroleum ether: ethyl acetate=10:1)) to obtain the 4-(3-N-methylindole)-nonyl -2-Kone liquid, pure product 115.3 mg, yield 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com