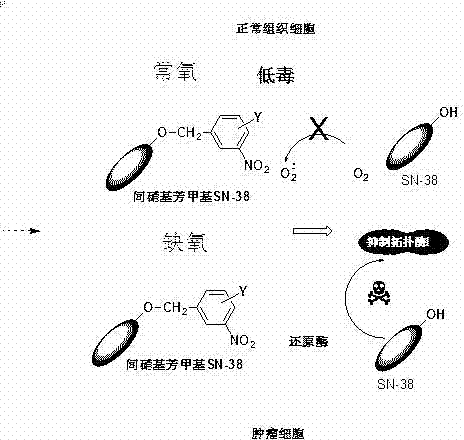
An m-nitroarylmethoxy camptothecin anoxic activation prodrug for antitumor drugs
A hypoxia-activated prodrug, nitrobenzyloxycamptotheca technology, used in antitumor drugs, drug combinations, organic chemistry, etc., can solve the problems of toxic side effects, unstable plasma metabolism, low bioavailability, etc. High selectivity, improved water solubility and stability, low toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1: Example of m-nitrobenzyl SN-38 and its preparation method
[0062] 1) The chemical name of m-nitrobenzyl SN-38 is:
[0063] (4S)-4,11-Diethyl-4-hydroxy-9-(3-nitrobenzyloxy)-1H-pyrano[3',4':6,7]indoleazino[1 ,2-b] quinoline-3,14(4H,12H)-dione;
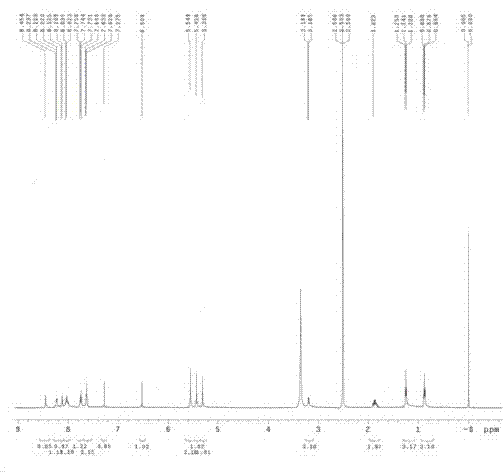
[0064] The structural formula is:
[0065]
[0066] 2) The preferred preparation method of m-nitrobenzyl SN-38 is as follows:
[0067] Dissolve 2.30 g of m-nitrobenzyl alcohol (3-nitrobenzyl alcohol) and 3.92 g of SN-38 in 40 ml of tetrahydrofuran, add 3.67 g of triphenylphosphine at room temperature, cool to 0°C, and dropwise add 2.78 g of azo After adding diethyl diformate, it was raised to room temperature, and after stirring for 3 hours, 200 ml of dichloromethane and 200 ml of water were added, and the organic phase was separated, and the aqueous layer was extracted 3 times with 200 ml of dichloromethane. The organic phases were combined and dried with anhydrous sodium sulfate, and the solvent was remove...
Embodiment 2
[0072] Example 2. The application effect of m-nitrobenzyl SN-38 and its comparison with the camptothecin derivative standard drug irinotecan
[0073] 1) Identification of anticancer activity of m-nitrobenzyl SN-38 and comparative analysis with irinotecan:
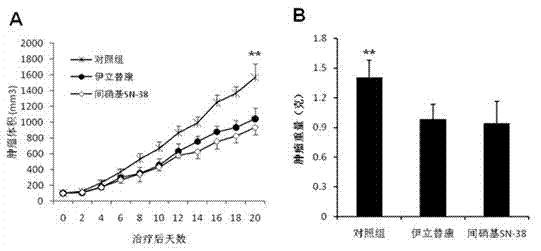
[0074] image 3It shows the growth of subcutaneous lung cancer A549 tumor in nude mice after treatment with m-nitrobenzyl SN-38 and irinotecan, and the comparison analysis with the growth of tumor in the control group.
[0075] 1 × 106 human lung cancer A549 cells in logarithmic growth phase were subcutaneously injected into the back of 6-week-old female Balb / c nude mice. When the tumor grew to 100 mm3 (day 0), the animals were randomly divided into three groups, namely the control group, irinotecan group and m-nitrobenzyl SN-38 group, and were given intraperitoneal injection of normal saline, irinotecan (50mg / kg, sorbitol / lactic acid buffer [45 mg / ml sorbitol / 0.9 mg / ml lactic acid] and m-nitrobenzyl SN-38 (50 mg / kg) eve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com