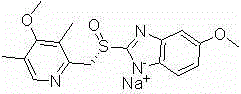
Esomeprazole sodium polymorph, preparation method and application thereof
A technology of esomeprazole sodium and polymorphs, applied in the field of medicinal chemistry, can solve the problems that impurities are difficult to reach 0.1%, solvents are difficult to meet the pharmacopoeia standards, and are not suitable for industrial production. The purity is good and the product purity is easy. Controlled, quality reproducible results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
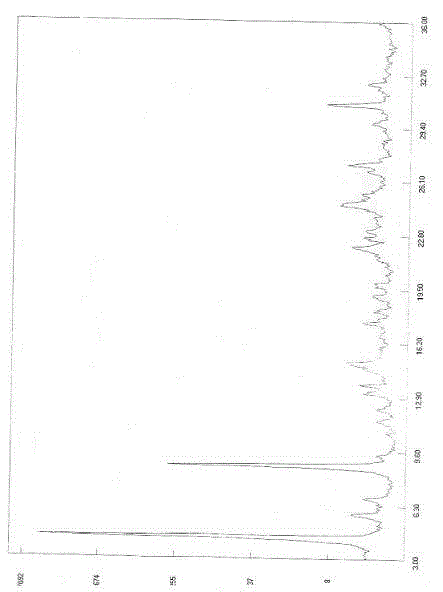
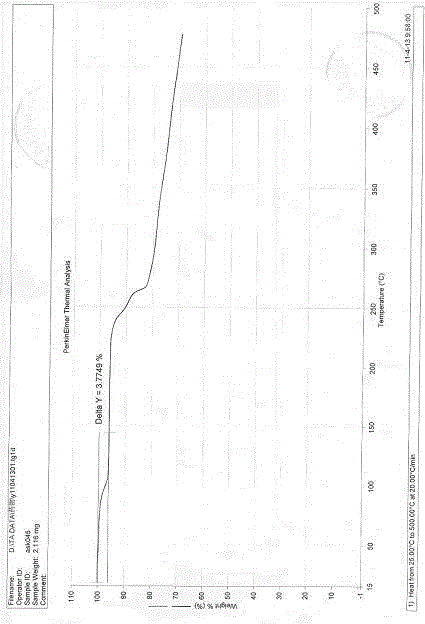
[0087] Embodiment 1: the preparation of esomeprazole sodium polymorph
[0088] Take 20g of esomeprazole and place it in a reaction flask, add 100mL of 2-butanone, stir until completely dissolved, filter, transfer the filtrate to the reaction flask, add 30% sodium hydroxide aqueous solution dropwise at an internal temperature of 20-25°C 8.1mL, after the dropwise addition, continue to stir the reaction at the same temperature for 5h, you can see that the color of the reaction becomes lighter. The reaction solution was concentrated under reduced pressure at 40°C, and the organic solvent was removed by concentration to obtain 40 g of a yellow oil. Slowly add 80g of diethyl ether to the yellow oil, crystallize at room temperature (21°C) for 3h, a large amount of white solid precipitates, then cool to 0°C for 1h to complete the crystallization. Suction filtration, the filter cake was washed with ether and dried. The solid was dried at 40°C under reduced pressure (-0.09MPa) to ob...
Embodiment 2
[0089] Embodiment 2: the preparation of esomeprazole sodium polymorph
[0090] Take 50g of esomeprazole and place it in a reaction flask, add 500mL of 2-butanone, stir well until it is completely dissolved, filter, transfer the filtrate to the reaction flask, and add 30% sodium hydroxide aqueous solution dropwise at an internal temperature of 10-15°C 20.3mL, after the dropwise addition, continue to stir and react at the same temperature for 10h. The reaction solution was concentrated under reduced pressure at 40°C, and the organic solvent was removed by concentration to obtain 52.6 g of a yellow oil. Slowly add 105.2 g of 2-butanone to the yellow oil, crystallize at room temperature (23°C) for 4 hours, and a large amount of white solid precipitates, then cool to 5°C and crystallize for 2 hours to complete the crystallization. Suction filtration, the filter cake was washed with 2-butanone and sucked dry. The solid was dried at 40°C under reduced pressure (-0.09MPa) to obtai...
Embodiment 3
[0091] Embodiment 3: the preparation of esomeprazole sodium polymorph
[0092] Take 5g of esomeprazole and place it in a reaction flask, add 40mL of 2-butanone, stir well until it is completely dissolved, filter, transfer the filtrate to the reaction flask, and add 30% sodium hydroxide aqueous solution dropwise at an internal temperature of 25-30°C 2.0mL, after the dropwise addition, continue to stir and react at the same temperature for 8h. The reaction solution was concentrated under reduced pressure at 40°C, and the organic solvent was removed by concentration to obtain 4.9 g of a yellow oil. Add 10 g of isopropyl ether to the yellow oil, cool to room temperature (20°C) and crystallize for 3 hours, a large amount of white solid precipitates, then cool to 10°C and crystallize for 2 hours to complete the crystallization. Suction filtration, the filter cake was washed with isopropyl ether and sucked dry. The solid was dried at 45°C under reduced pressure (-0.09MPa) to obta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com