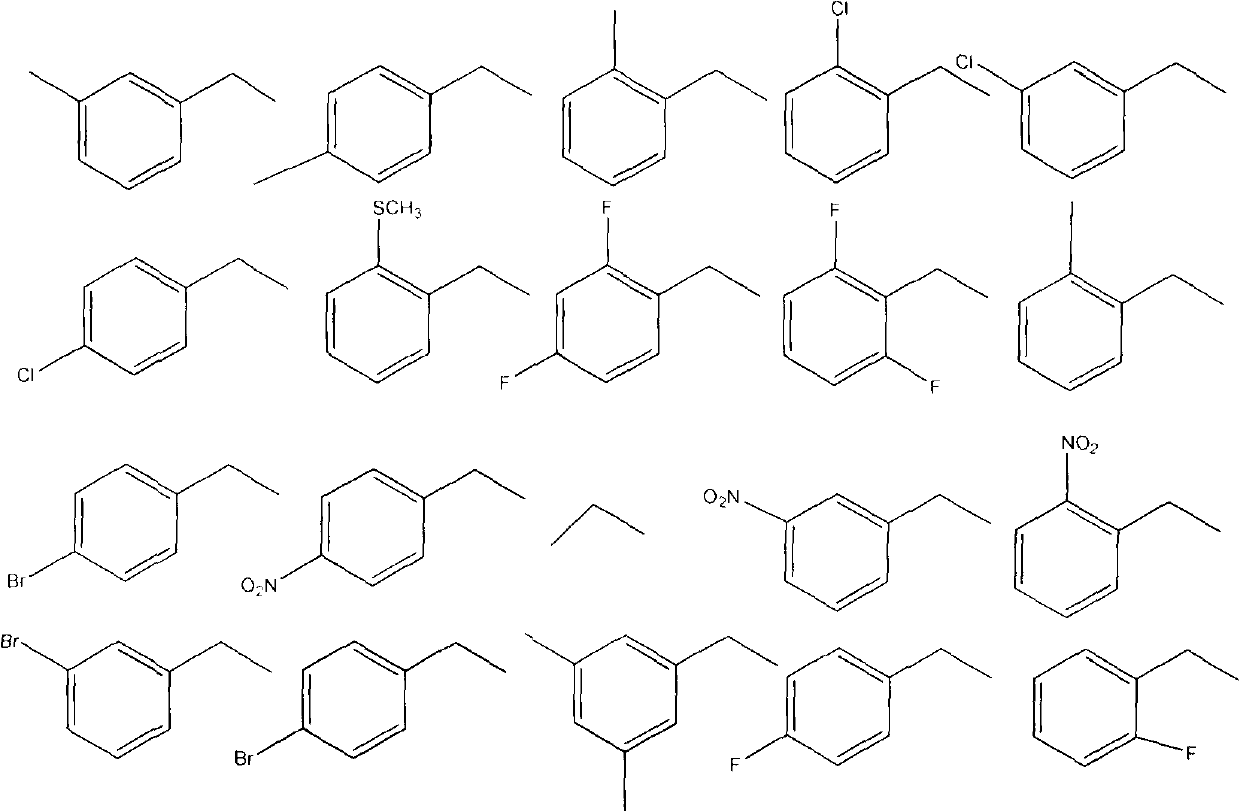
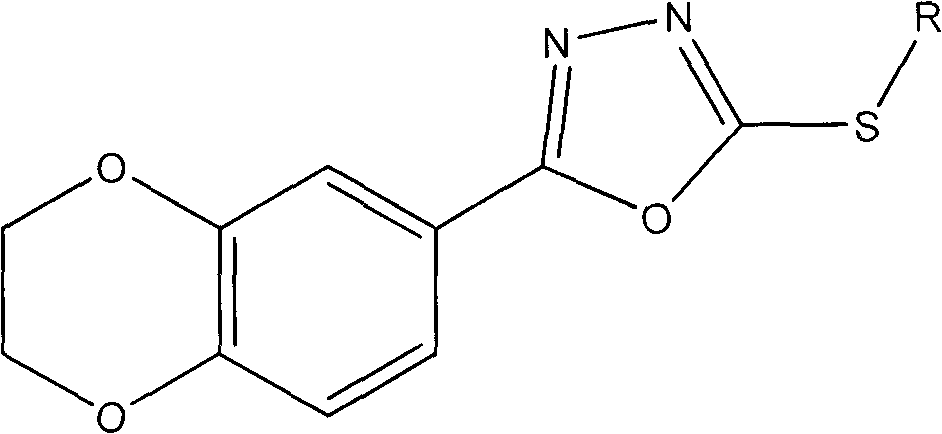
Preparation method of 1,4-benzodioxan-containing 1,3,4-oxadiazole derivatives and use of the 1,4-benzdioxan-containing 1,3,4-oxadiazole derivatives in anti-cancer drugs
A technology of benzodioxane and oxadiazole, applied to the preparation method of 1,3,4-oxadiazole derivatives containing 1,4-benzodioxane and its application field in anticancer drugs , which can solve problems such as application restrictions, many adverse reactions, and limited efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: 2-(2,3-dihydrobenzo[b][1,4]dioxan-6-yl)-5-(3-methylbenzyl)-1,3,4-oxa Preparation of Oxadiazole (Compound 1).
[0021]
[0022] 1 mmol of 1,4-benzodioxane-6-carboxylic acid was subjected to esterification under the catalysis of concentrated sulfuric acid to obtain the corresponding ester. Then dissolve the obtained ester in an appropriate amount of methanol, add a slight excess of 85% hydrazine hydrate, and stir and reflux for 8-12 hours. The solvent was evaporated under reduced pressure, and then water was added. After the solid precipitated, it was filtered and washed with water, and recrystallized from ethanol to obtain a substituted hydrazide. After dissolving 1mmol hydrazide and 1mmol KOH in an appropriate amount of 95% ethanol, slowly add a little excess CS 2 Stir and reflux, evaporate the solvent ethanol after the reaction is terminated, pour it into cold water, adjust the pH value to 5-6 with dilute hydrochloric acid, produce a large amount of pre...
Embodiment 2
[0023] Example 2: 2-(2,3-dihydrobenzo[b][1,4]dioxan-6-yl)-5-(4-methylbenzyl)-1,3,4-oxa Preparation of Oxadiazole (Compound 2).
[0024]
[0025] The preparation method is the same as in Example 1. Substitute 4-methylbenzyl bromide for 3-methylbenzyl bromide to obtain the target compound. Yellow needle-like crystals, yield 76%; m.p.133-1°C; 1 H NMR (500MHz, CDCl 3 )δ: 3.4(m, 3H), 4.29-4.32(m, 4H), 4.47(s, 2H), 6.94(d, J=8.25Hz, 1H), 7.02(t, J=8.55, 2H) 7.33(d , J=7.9, 2H) 7.48 (d, J=9.15, 2H); MS (ESI): 341.09 (C 18 h 17 N 2 o 3 S, [M+H] + ).Anal.Calcd for C 18 h 16 N 2 o 3 S: C, 63.51; H, 4.74; N, 8.23%. Found: C, 63.36; H, 4.47; N, 8.48%.
Embodiment 3
[0026] Example 3: 2-(2,3-dihydrobenzo[b][1,4]dioxan-6-yl)-5-(2-fluorobenzyl)-1,3,4-oxadi Preparation of azole (compound 3).
[0027]
[0028] The preparation method is the same as in Example 1. Substitute 2-fluorobenzyl bromide for 3-methylbenzyl bromide to obtain the target compound. Pale yellow crystals. Yield 79.5%; mp: 108°C. 1 H NMR (500MHz, CDCl 3 )δ: 4.28-4.31 (m, 4H), 4.52 (s, 2H), 6.94 (d, J=8.2, 1H), 7.04-7.11 (m, 2H), 7.28 (d, J=7.8, 1H) 7.46 -7.49 (m, 2H), 7.52 (q, J=6.25, 1H); MS (ESI): 345.06 (C 17 h 14 N 2 o 3 S, [M+H] + ).Anal.Calcd for C 17 h 13 N 2 o 3 S: C, 59.29; H, 3.81; N, 8.13%. Found: C, 59.40; H, 3.82; N, 8.03%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com