Method for simultaneously determining three alkaloids in granules for eliminating phlegm and stopping cough for children
A technology for resolving phlegm, relieving cough, and measuring methods, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., and can solve the problems of unchecked determination of epecoline and emetine, poor accuracy, environmental pollution, etc., and achieve separation Good, fast filtration speed, less impurity components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
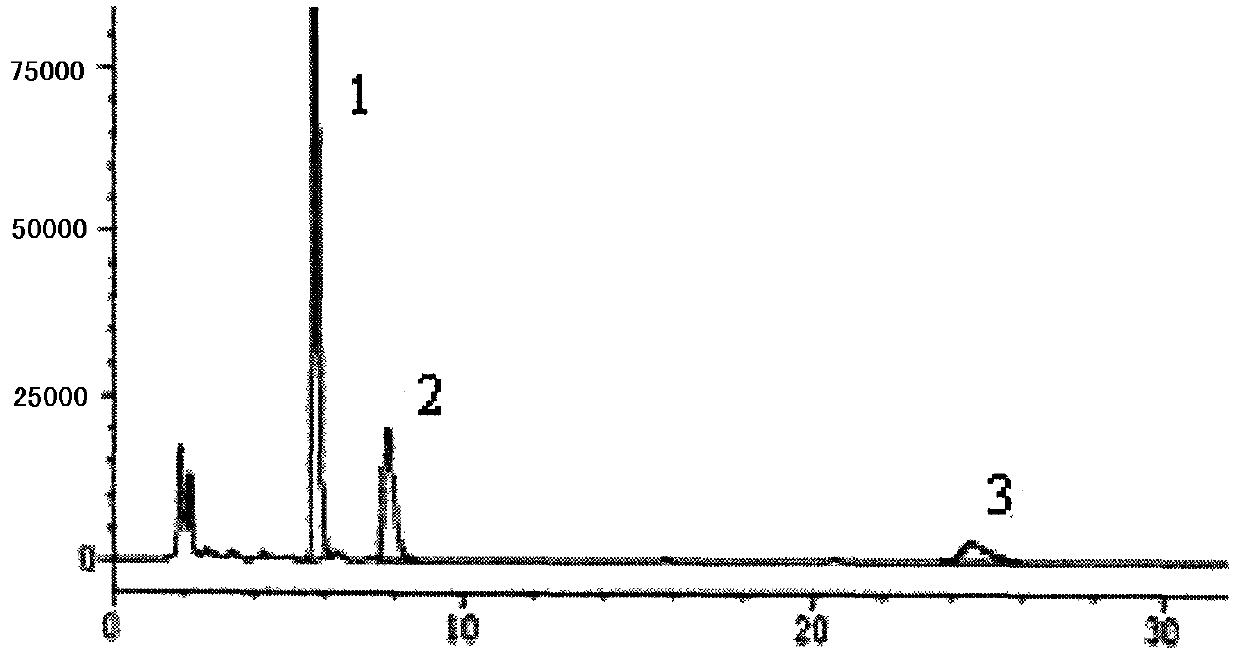
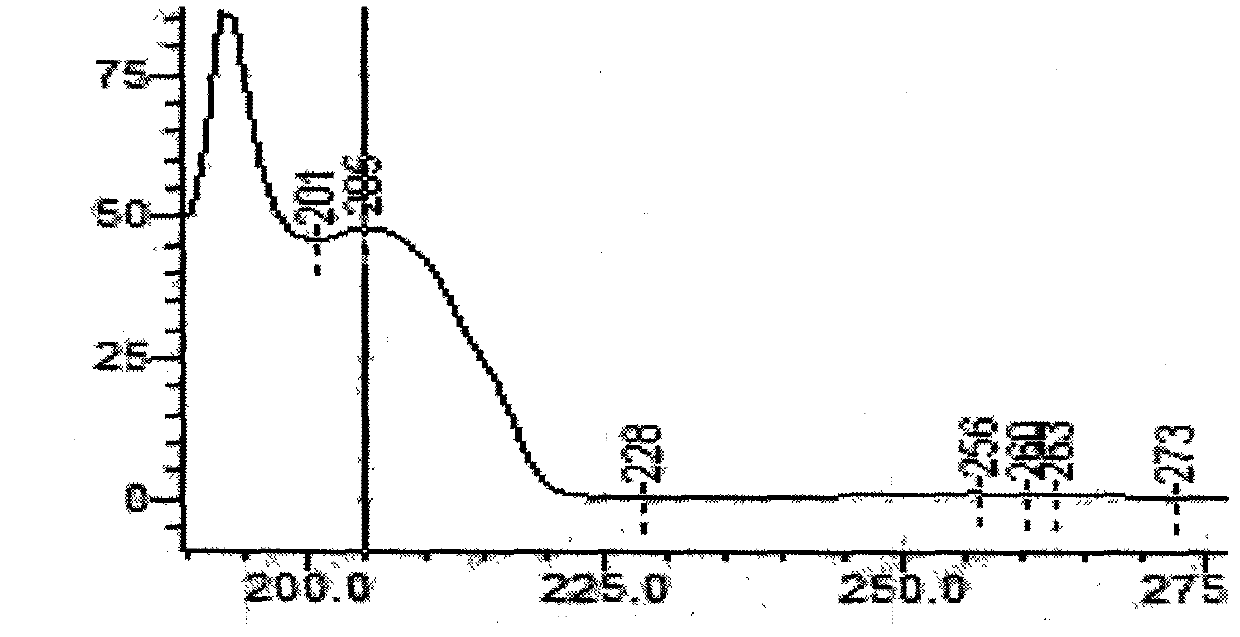
[0037] 1. Chromatographic conditions and system suitability test Acetonitrile-methanol-0.1% phosphoric acid with a volume ratio of 1.5:12.5:86 was used as the mobile phase; column temperature: 40°C; detection wavelength was 205nm; the number of theoretical plates was calculated according to the emetine peak Should not be less than 1200;
[0038] 2. Preparation of reference substance solution Take appropriate amount of ephedrine hydrochloride, emetecine hydrochloride and emetine hydrochloride reference substance, accurately weigh them, put them in a brown measuring bottle, add methanol to make stock solution, then take the stock solution, add mobile phase to prepare Become the solution that every 1ml contains ephedrine hydrochloride 50 μ g, emetylline hydrochloride and emetine hydrochloride respectively 4 μ g, to obtain final product;
[0039] 3. Preparation of the test solution Take an appropriate amount of this product, grind it finely, weigh 1 / 2 of the packaged amount of 3g ...
Embodiment 2
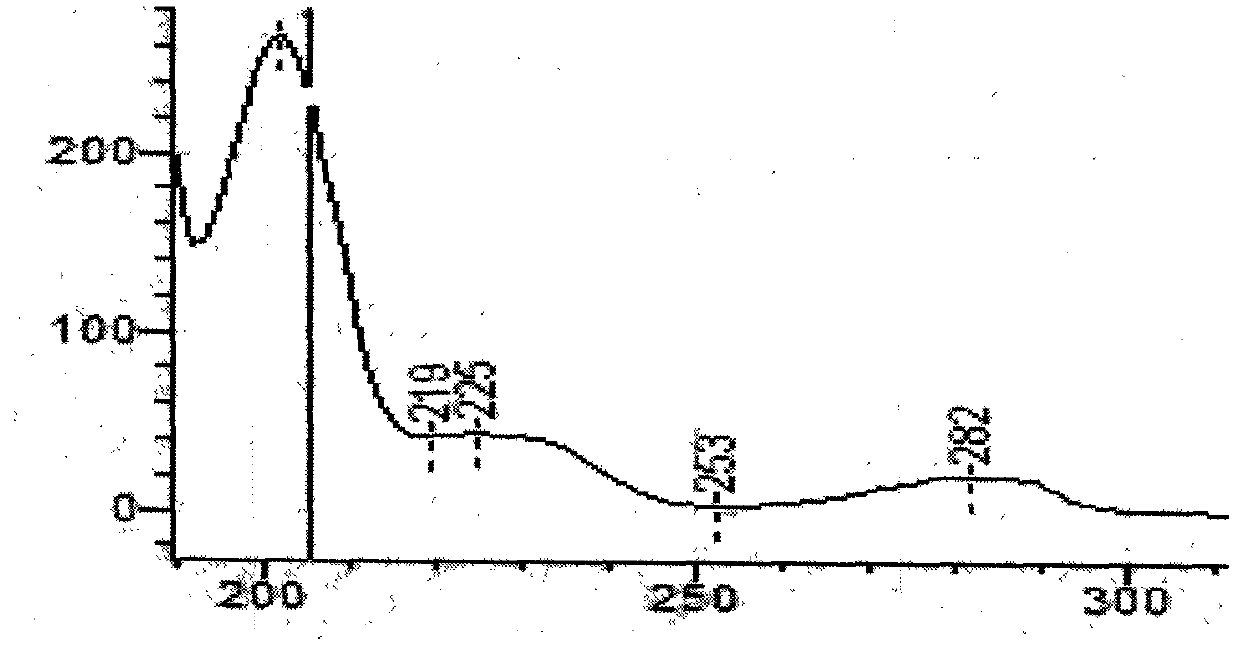
[0042] 1. Chromatographic conditions and system suitability test: Acetonitrile-methanol-0.1% phosphoric acid with a volume ratio of 2:11.5:86.5 is used as the mobile phase; column temperature: 40°C; detection wavelength is 205nm; the number of theoretical plates is calculated according to the emetine peak Should not be less than 1200;
[0043] 2. Preparation of reference substance solution Take appropriate amount of ephedrine hydrochloride, emetecine hydrochloride and emetine hydrochloride reference substance, accurately weigh them, put them in a brown measuring bottle, add methanol to make stock solution, then take the stock solution, add mobile phase to prepare Become the solution that every 1ml contains ephedrine hydrochloride 50 μ g, emetylline hydrochloride and emetine hydrochloride respectively 4 μ g, to obtain final product;
[0044] 3. Preparation of the test solution Take an appropriate amount of this product, grind it finely, weigh 1 / 2 of the packaged amount of 4g pe...
Embodiment 3
[0047] 1. Chromatographic conditions and system suitability test: Acetonitrile-methanol-0.1% phosphoric acid with a volume ratio of 1.0:12.5~11:86.5 is used as the mobile phase; column temperature: 40°C; detection wavelength is 205nm; the number of theoretical plates is based on emetine Peak calculation should not be lower than 1200;
[0048] 2. Preparation of reference substance solution Take appropriate amount of ephedrine hydrochloride, emetecine hydrochloride and emetine hydrochloride reference substance, accurately weigh them, put them in a brown measuring bottle, add methanol to make stock solution, then take the stock solution, add mobile phase to prepare Become the solution that every 1ml contains ephedrine hydrochloride 50 μ g, emetylline hydrochloride and emetine hydrochloride respectively 4 μ g, to obtain final product;
[0049] 3. Preparation of the test solution Take an appropriate amount of this product, grind it finely, weigh 1 / 2 of the packaging volume of 5g pe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com