Method for recovering rare earth from waste rare earth luminescent material
A rare earth luminescence and waste technology, which is applied in the direction of luminescent materials, lamp tube/lamp material recycling, electronic waste recycling, etc., can solve the problems of Al impurity element removal, mercury pollution, etc., achieve high recovery rate, solve environmental pollution, avoid pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
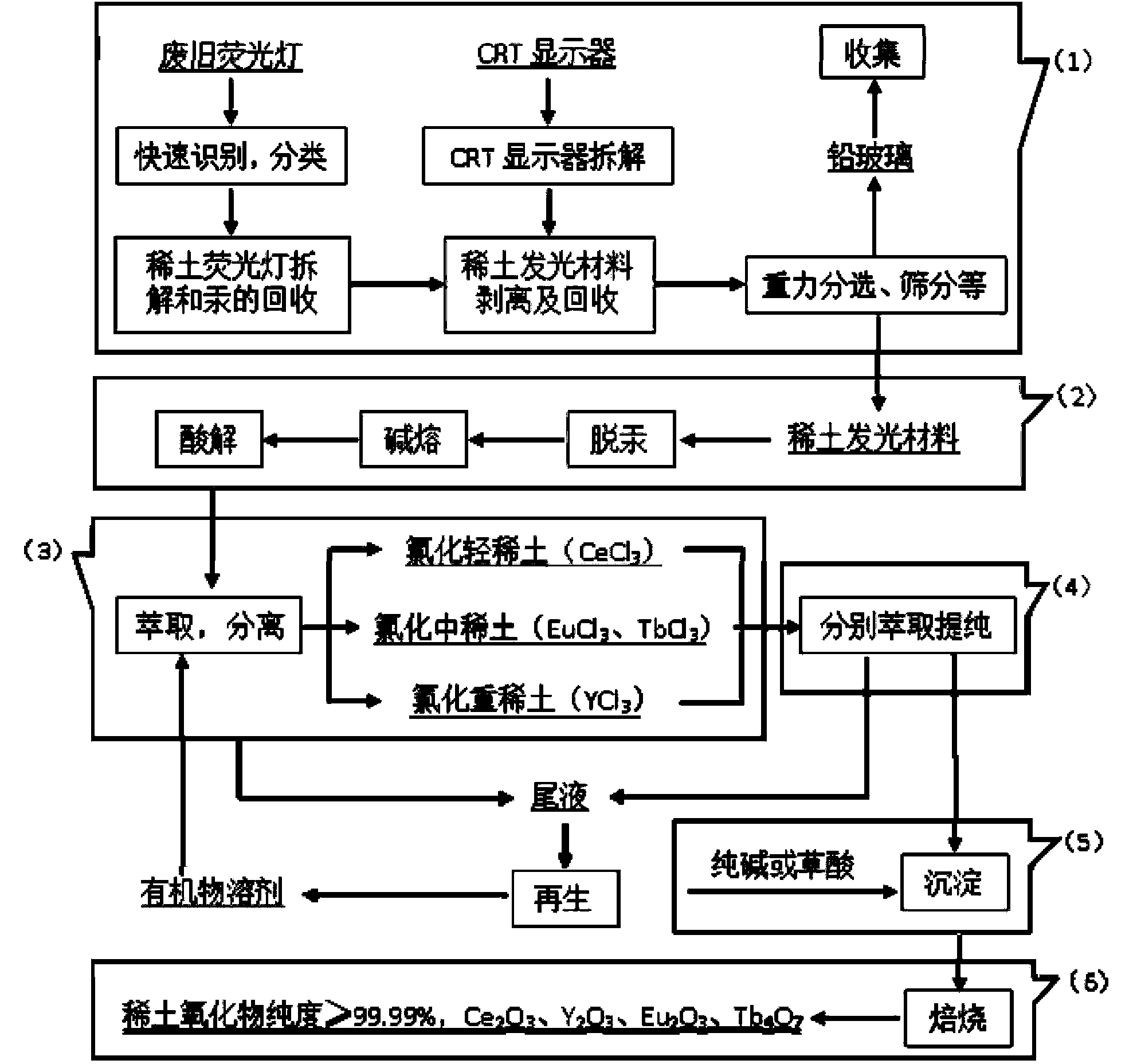
[0054] Discarded fluorescent lamps and CRT monitors were dismantled and broken, and mercury vapor was collected under negative pressure. The concentration of waste fluorescent powder is 20 wt % of acetone solution for cleaning, using a concentration of 0.8g / L potassium permanganate for oxidative demercuration for 4 hours, the addition of zinc sulfide is 0.8g / L cleaning solution, through sulfide precipitation Hg 2+ , and finally the residual mercury in the phosphor is removed by activated carbon.
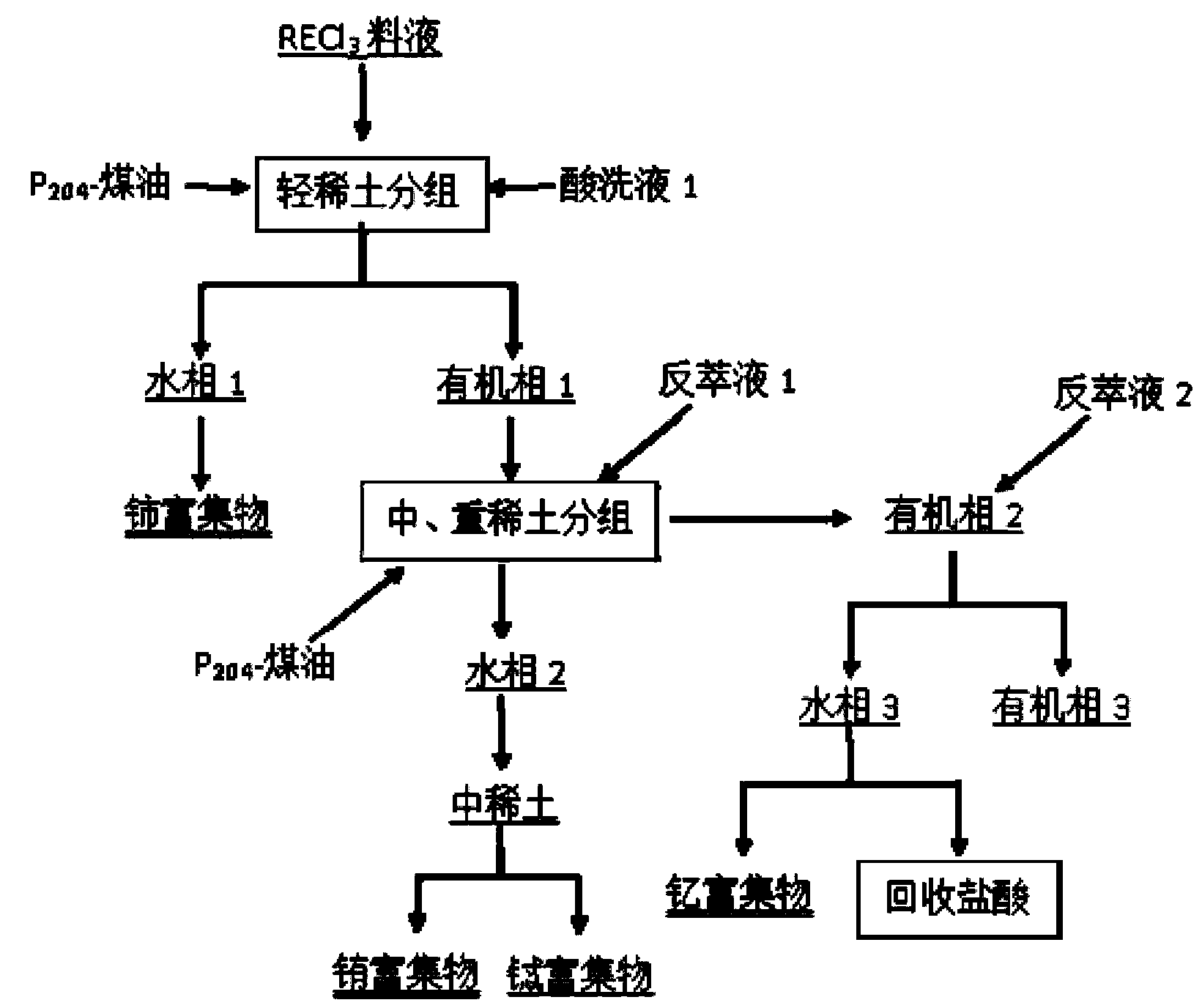
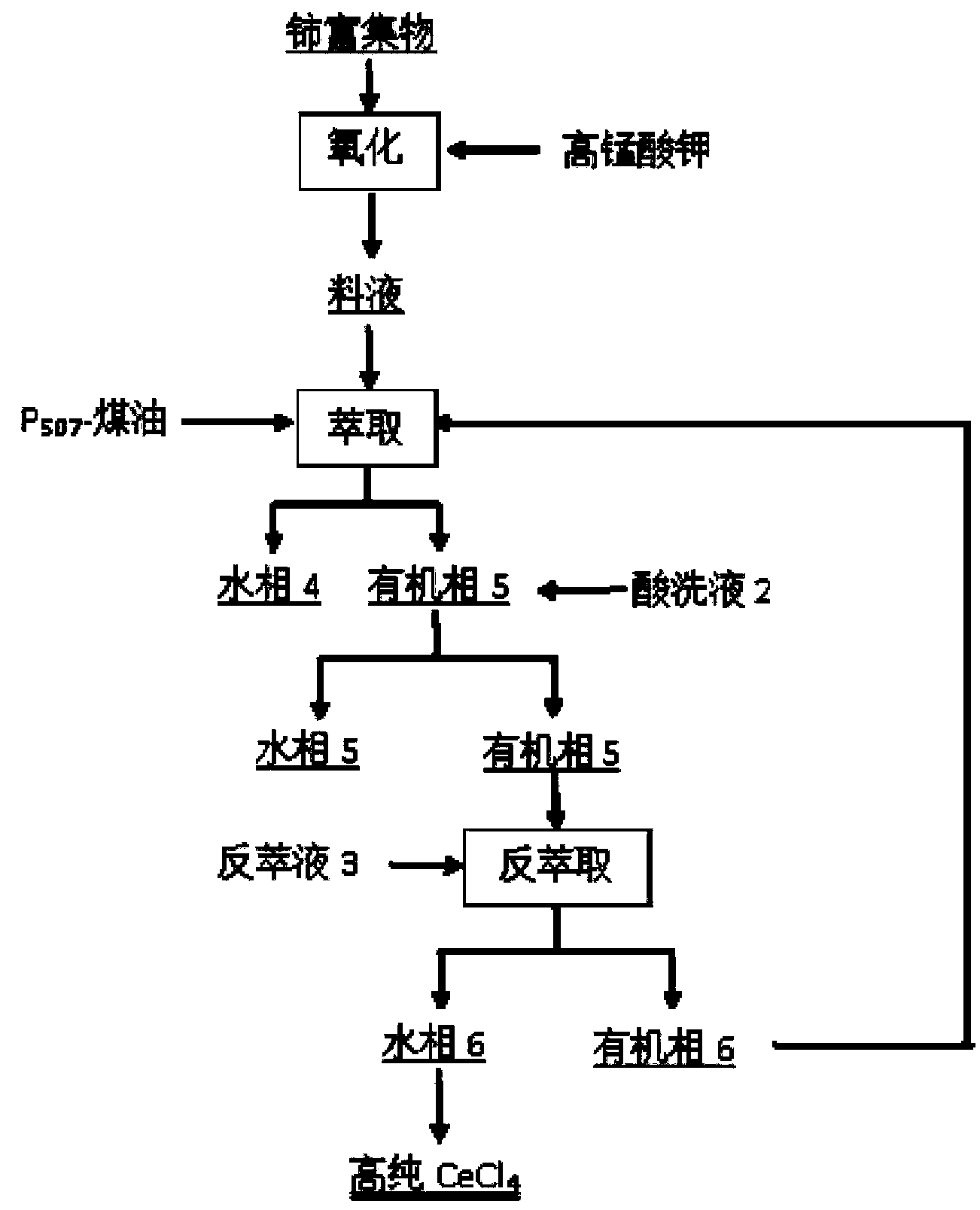
[0055] Mix and stir the cleaned waste rare earth luminescent material and alkali (NaOH) evenly, add water and stir evenly at a mass ratio of 1:6, then alkali-melt the product obtained after alkali melting at 800°C for 1 hour, and wash with deionized water twice Remove remaining NaOH and part of NaAlO 2 Insolubles containing rare earths were obtained. Use 6mol / L hydrochloric acid to carry out acidolysis at 70°C for 2 hours. The solid-to-liquid ratio of insoluble matter to hydrochlo...
Embodiment 2
[0063] Discarded fluorescent lamps and CRT monitors were dismantled and broken, and mercury vapor was collected under negative pressure. The concentration of waste fluorescent powder is 30 wt % of acetone solution, and use potassium permanganate concentration of 1g / L for oxidative mercury removal for 5 hours to remove residual mercury in the phosphor.
[0064] Mix and stir the cleaned waste rare earth luminescent material and alkali (KOH) evenly, add water and stir evenly at a mass ratio of 1:10, and then perform alkali fusion at 1000°C for 3 hours to obtain the alkali fusion product, and wash it with deionized water twice Removal of remaining KOH and part of KAlO 2 Insolubles containing rare earths were obtained. Use 8mol / L hydrochloric acid to carry out acidolysis at 60°C for 4 hours. The solid-to-liquid ratio of insolubles to hydrochloric acid is 1:3. The pH of the acidolysis solution is adjusted to 4 with ammonia water, and 5 wt % of PAC flocculant to remove Al in acid ...
Embodiment 3
[0072] Discarded fluorescent lamps and CRT monitors were dismantled and broken, and mercury vapor was collected under negative pressure. The concentration of waste fluorescent powder is 10 wt % acetone solution, and use 0.2g / L potassium permanganate for oxidative demercuration for 0.5h to remove the residual mercury in the fluorescent powder.
[0073] Mix and stir the cleaned waste rare earth luminescent material and alkali (NaOH) evenly, add water at a mass ratio of 1:2 and stir evenly, then alkali-melt the product obtained after alkali melting at 1200°C for 5 hours, and wash it with deionized water twice Remove remaining NaOH and part of NaAlO 2 Insolubles containing rare earths were obtained. Use 3mol / L hydrochloric acid to carry out acidolysis at 80°C for 6 hours, the solid-to-liquid ratio of insoluble matter to hydrochloric acid is 1:5, the pH of the acidolysis solution is adjusted to 5 with ammonia water, add 2 wt % of PAC flocculant to remove Al in acid hydrolysis so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com