Phenothiazinyl quinazoline fluorescence ion probe and application thereof
A phenothiazinyl quinazoline, fluorescent ion technology, applied in the field of fluorescent ion probes, can solve problems such as hindering probe sensitivity, and achieve the effects of high sensitivity and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1, the preparation of compound ECQP:
[0023] Weigh 2.85 g (14.6 mmol) 9-ethylcarbazole in a three-necked flask, N 2 Add 16 mL DMF and 20 mL 1,2-dichloroethane under protection, and add 16 mL (175 mmol, ρ=1.675) POCl dropwise from the constant pressure funnel under stirring 3 , the dropwise addition was completed, the temperature was raised to 80 °C for 8 h, cooled, the reaction solution was poured into water, neutralized with dilute ammonia water to weak acidity, extracted with dichloromethane, purified by silica gel chromatography, and recrystallized from ethanol to obtain 9-ethyl- 2.82 g of 3-formyl-9H-carbazole is a pale yellow solid. Yield: 86.6%. m.p.: 84-85 o c. 1 H NMR (CDCl 3 , 400MHz) δ (ppm): 1.43-1.47 (t, 3H), 4.34-4.40 (m, 2H), 7.31-7.34 (t, 1H), 7.43-7.46 (d, 2H), 8.52-7.56 (t, 1H), 7.98-8.08 (d, 1H), 8.13-8.15 (d, 1H), 8.58 (s, 1H), 10.08 (s, 1H). GC MS (m / z): 223 (M + ).
[0024] Weigh 1.56 g (7 mmol) 9-ethyl-3-formyl-9H-carbazole, 0.95...
Embodiment 2
[0028] Embodiment 2, ECQP ultraviolet absorption spectroscopic titration experiment:
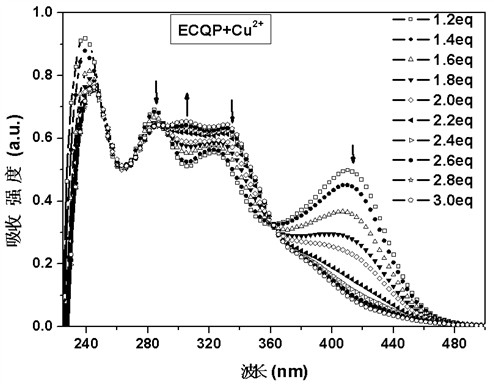
[0029] Compound ECQP was formulated as 2 × 10 -5 mol / L dichloromethane solution, pipette 2.5 mL of the prepared compound ECQP solution in a fluorescence cuvette, and drop 5 μL 1×10 -3 mol / L of Cu 2+ solution in acetonitrile until equilibrium is reached (ie, the absorption spectrum no longer changes appreciably). Without adding Cu 2+ , there were three absorption peaks in the absorption spectrum of ECQP, among which the absorption peak at 245 nm belonged to the characteristic absorption of quinazoline, while the absorption around 300 nm and 344 nm belonged to the characteristic absorption of carbazole and phenothiazine, respectively. When adding 0~1 eq Cu 2+ A new absorption peak appeared at 410 nm, and with Cu 2+ The addition of increased gradually, indicating that the addition of Cu 2+ Formed a complex with ECQP (as shown in the accompanying drawing figure 1 shown). When continui...
Embodiment 3
[0030] Embodiment 3, ECQP fluorescence spectrum titration experiment:
[0031] Compound ECQP was formulated as 2 × 10 -5 mol / L dichloromethane solution, pipette 2.5 mL of the prepared compound ECQP solution in a fluorescence cuvette, and drop 5 μL 1×10 -3 mol / L of Cu 2+ solution in acetonitrile until equilibrium is reached (i.e., the spectrum no longer changes appreciably). Without adding Cu 2+ , ECQP does not emit light in dichloromethane solution, with Cu 2+ (0~3 eq) of acetonitrile solution, an emission peak appeared at 504 nm, and with Cu 2+ The addition gradually increased. It can be observed that adding 0~1 eq Cu 2+ When , the emission peak intensity increases slowly, while adding 1~3 eq Cu 2+ When , the intensity increases rapidly, and reaches the maximum at 3 eq (as shown in the accompanying drawing image 3 shown).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com