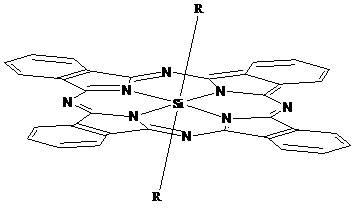
Phthalocyanine silicon modified by amino ethyl groups and phenoxy groups as well as preparation method and application thereof
A technology of aminoethylphenoxy and phthalocyanine silicon, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, active ingredients of silicon compounds, etc., and can solve complex synthetic routes, clinical application limitations, and high skin phototoxicity, etc. problems, to achieve the effects of low preparation cost, easy separation and purification, and clear structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Synthesis and physical and chemical properties of bis[4-(2-aminoethyl)phenoxy]phthalocyanine silicon (IV) (structure shown in the following formula):
[0036]
[0037] Under nitrogen protection, dichlorosilyl phthalocyanine (244.7mg, 0.4mmol), 4-(2-aminoethyl)phenol 1.2~2 mmol (preferably 1.6mmol) and NaH were added to toluene or xylene or dioxane In 20~50ml (preferably toluene, 30ml), reflux for 12~24 hours (preferably 18 hours). Remove the solvent by rotary evaporation in vacuo, dissolve in 100ml of dichloromethane, centrifuge to remove insoluble matter, extract the dichloromethane solution with water (3×100ml), collect the organic layer, then extract with dilute hydrochloric acid (0.1-0.5 mmol), and collect the aqueous layer. The aqueous layer was neutralized with 1M sodium hydroxide, and a blue precipitate was precipitated, centrifuged, washed with water, and dried in vacuum to obtain a blue product with a yield of 45%. The maximum absorption peak of the product...
Embodiment 2
[0040] Synthesis and physicochemical properties of bis[4-(2-aminoethyl)phenoxy]phthalocyanine silicon (IV) hydrochloride (structure shown in the following formula):
[0041]
[0042] Under nitrogen protection, add dichlorosilicon phthalocyanine (305.8mg, 0.5mmol), 4-(2-aminoethyl)phenol 1.5~2.5mmol (preferably 2mmol) and NaH to toluene 30~60ml (preferably 40ml) , Reflux for 12-24 hours (preferably 18 hours). Remove toluene by vacuum rotary evaporation, dissolve in 120ml of dichloromethane, centrifuge to remove insoluble matter, extract the dichloromethane solution with water (3×100ml), collect the organic layer, then extract with dilute hydrochloric acid (0.1-0.5 mmol), and collect the aqueous layer. Add aqueous sodium chloride solution (preferably saturated aqueous sodium chloride solution) to the water layer to precipitate a blue precipitate, centrifuge, wash with water, and dry under vacuum at room temperature to obtain a blue product with a yield of 50%. The maximum ab...
Embodiment 3
[0045] The method for preparing photodynamic drugs (i.e. photosensitizers) using silicon phthalocyanine described in the present invention is: use water, or a mixed solution of water and other substances (the content of other substances is not higher than 10% (wt%)) as a solvent , dissolving silicon phthalocyanine of the present invention, and preparing a uniform blue solution (ie photosensitizer), the concentration of silicon phthalocyanine in the photosensitizer is 0.08mM. The other substances mentioned can be one or a combination of the following: castor oil derivatives (Cremophor EL), dimethyl sulfoxide, ethanol, glycerin, N,N-dimethylformamide, polyethylene glycol 300- 3000, Cyclodextrin, Dextrose, Tween, Macrogol Monostearate. The silicon bis[4-(2-aminoethyl)phenoxy]phthalocyanine of the present invention can also be converted into a salt form with hydrochloric acid or sulfuric acid or other acidic substances, and then dissolved in the above-mentioned solvent. In the pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com