3-methylquinoxaline-2-carboxylic acid artificial antigen and antibody obtained by the 3-methylquinoxaline-2-carboxylic acid artificial antigen
A technology of methylquinoxaline and carboxylic acid, applied in the field of 3-methylquinoxaline-2carboxylic acid artificial antigen and its prepared antibody, can solve the problems of carcinogenesis, endangering human and animal health, and mutagenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1, preparation 3-methylquinoxaline-2-carboxylic acid hapten
[0030] One, the preparation of 3-methylquinoxaline-2-carboxylic acid hapten
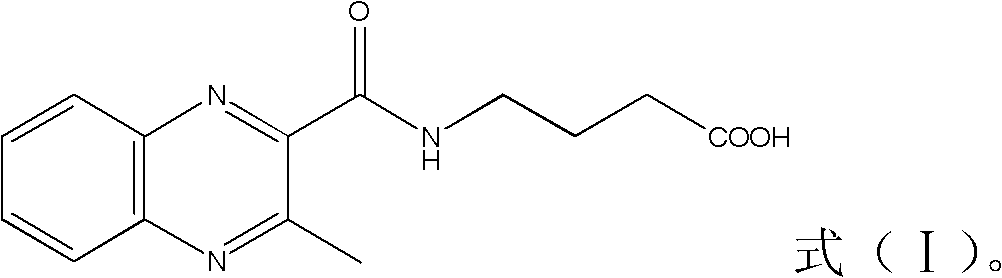
[0031] 1. Weigh 10 mg of 3-methylquinoxaline-2-carboxylic acid and place it in a 10mL reaction bottle; add an appropriate amount of acetone and a small amount of DMF to completely dissolve it (acetone is used as a reaction system to dissolve 3-methylquinoxaline-2 -Carboxylic acid, the role of DMF is to promote dissolution), add 10 μL of thionyl chloride (acting as activated carboxyl group), heat and reflux for 1 h; then add 0.5 ml of n-hexane and blow it with nitrogen until the volume is constant, then add 0.5 ml of n-hexane and blow it with nitrogen Blow until the volume is constant, then add 0.5 ml of n-hexane and blow with nitrogen until the volume is constant to obtain solution I.
[0032] 2. Weigh 20mg of γ-aminobutyric acid, dissolve it in 1ml of 2mol / L KOH aqueous solution (as a reaction system to dissolve γ-amino...
Embodiment 2
[0039] Preparation and characterization of embodiment 2, 3-methylquinoxaline-2-carboxylic acid artificial antigen
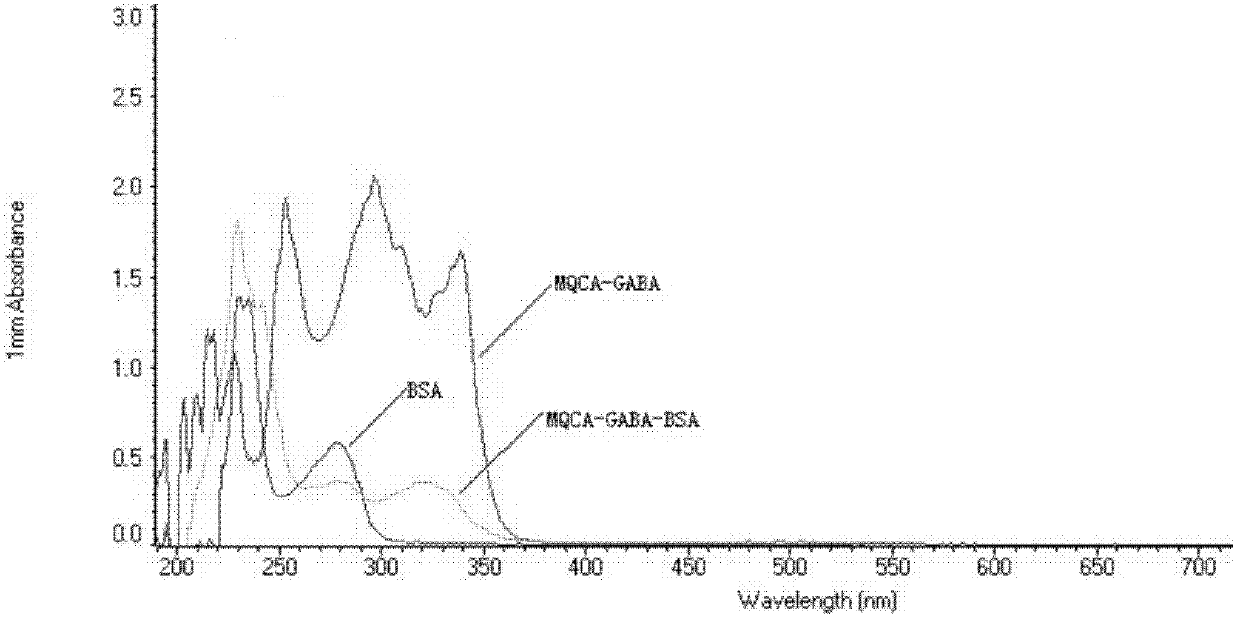
[0040] 1. Synthesis and characterization of 3-methylquinoxaline-2-carboxylic acid immunogen
[0041] 1. Preparation of 3-methylquinoxaline-2-carboxylic acid immunogen
[0042] (1) Dissolve 10 mg of the compound shown in formula (I) prepared in Example 1 in 2 mL of N, N'-dimethylamide, add 10 mg of N-hydroxysuccinimide and 10 mg of 1-ethyl-(3 -Dimethylaminopropyl) carbodiimide hydrochloride, magnetically stirred at room temperature for 2 h to obtain solution III.
[0043] (2) Add 30mg of bovine serum albumin into 2mL of PBS buffer solution and fully dissolve to obtain solution IV.
[0044] (3) Slowly add solution III to solution IV, stir slowly for 24 hours, put it into a dialysis bag, dialyze in normal saline at 4°C for 72h (change the water 6 times in the middle), then centrifuge at 8000rmp for 30min at 4°C, and take The supernatant, that is, the 3-methylquin...
Embodiment 3
[0058] Preparation of embodiment 3, 3-methylquinoxaline-2-carboxylic acid monoclonal antibody
[0059] Balb / c mice: purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.;
[0060] SP2 / 0 myeloma cells: purchased from Siggma-Aldrich Company, the product catalog number is 08060101.
[0061] 1. Animal immunity
[0062] The MQCA-BSA solution prepared in Example 2 was used to immunize Balb / c mice, and each mouse was immunized with 100 μg MQCA-BSA once, for a total of 4 times with an interval of two weeks between each time. Injection, the last three immunizations were intraperitoneal injection.
[0063] 2. Cell fusion and cloning
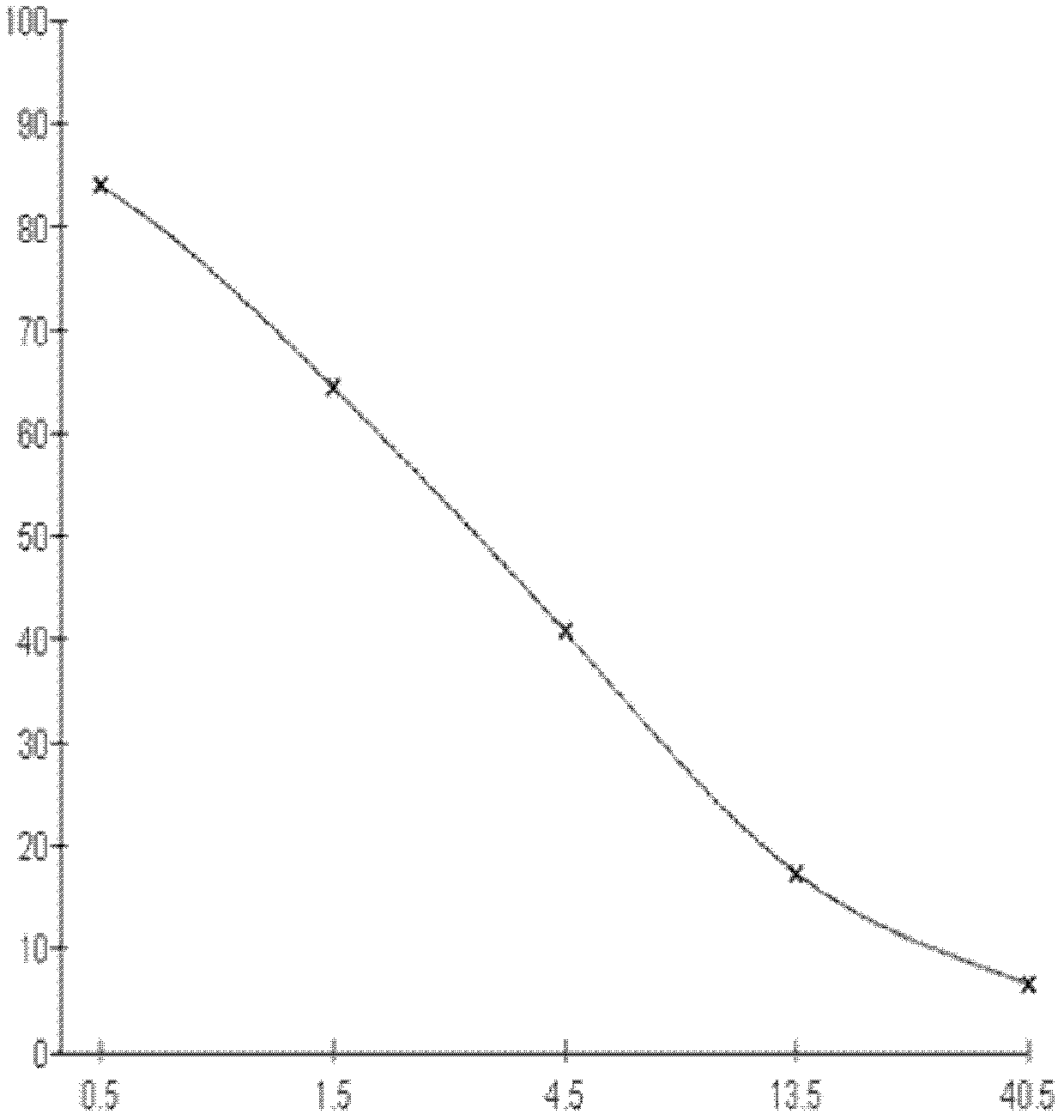
[0064] 1. Three days after the fourth immunization, splenocytes were collected and fused with SP2 / 0 myeloma cells at a ratio of 5:1 (quantity ratio). Cell supernatants were measured by indirect competitive ELISA, and positive wells were screened.
[0065] 2. Cloning the positive wells by using the limiting dilution method to obt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com