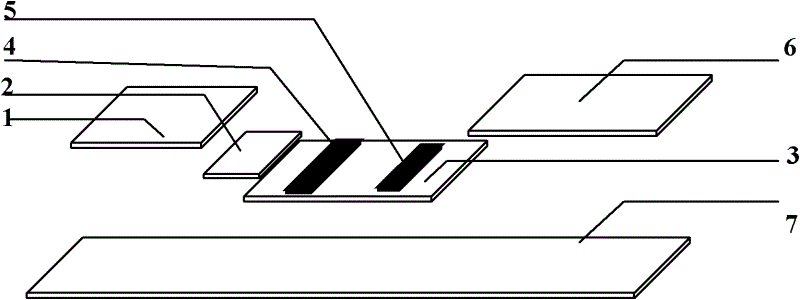
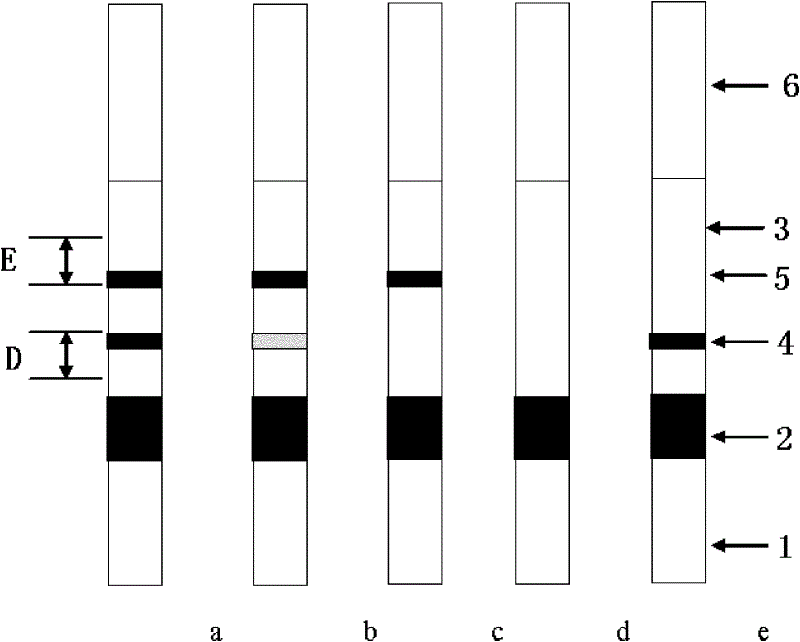
Colloidal gold chromatography anti-Ro52 antibody detection test paper and preparation method thereof
A technology of antibody detection and colloidal gold, which is applied in the field of medical immunization and can solve the problems that colloidal gold immunization test paper has not come out.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1 Preparation of RO52 Antigen Protein
[0071] The RO52 antigen protein used in this test paper is constructed by gene cloning technology to construct recombinant gene, and then the RO52 antigen protein which is all human origin is successfully expressed by prokaryotic expression technology. Wherein, the RO52 antigen protein is derived from purified genetic engineering antigen protein.
Embodiment 2
[0072] Example 2 Antibody Preparation
[0073] Antibody A, Antibody C, and Antibody B were prepared by the following method. Anti-human IgG in Antibody A, Antibody C and Antibody B can generally be produced by multiple subcutaneous (sc) or intraperitoneal (ip) injections of purified immunogens and adjuvants on animals.
[0074] The injection solution was obtained by mixing 0.05mg~1mg of immune preparations (respectively for goats or mice) with 3 times the volume of Freund's complete adjuvant, and injected the injection solution into multiple parts of the animal skin. 5 to 1 / 10 of the original amount of human IgG in Freund's complete adjuvant mixture was injected subcutaneously into multiple sites to boost the immunization. After 7-14 days, the animals were bled, and the serum anti-human IgG titer was measured. Animals were boosted until titers plateaued. Methods for producing polyclonal antibodies are described in many immunology textbooks, for example, "Common Experimental...
Embodiment 3
[0077] Colloidal gold solution preparation
[0078] 0.01% HAuCl 4 Heat the solution to boiling, quickly add every 100mL HAuCl 4 Add an appropriate amount of reducing agent solution to the solution, and the color changes from blue, then light blue, to blue, then red after heating, and transparent orange-red after boiling for 7-10 minutes. Then filter with ultrafiltration or microporous membrane (0.45μm) to remove the polymer and other impurities that may be mixed therein. The prepared colloidal gold should be pure, translucent, free of sediment and floating matter, and discard when oily matter and a large amount of black granular precipitate appear on the liquid surface.
[0079] Wherein the reducing agent used can be trisodium citrate (Frens 1973), tannic acid-trisodium citrate (Slot and Gueeze 1985), white phosphorus, preferably use trisodium citrate, more preferably use 1% trisodium citrate sodium.
[0080] The glass containers used should be absolutely clean, and must b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com