Human serum albumin nano biomaterial and preparation method thereof
A technology of human serum albumin and nanofibers, applied in the direction of spinning solution preparation, single-component protein rayon, application, etc., to achieve the effect of simple production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
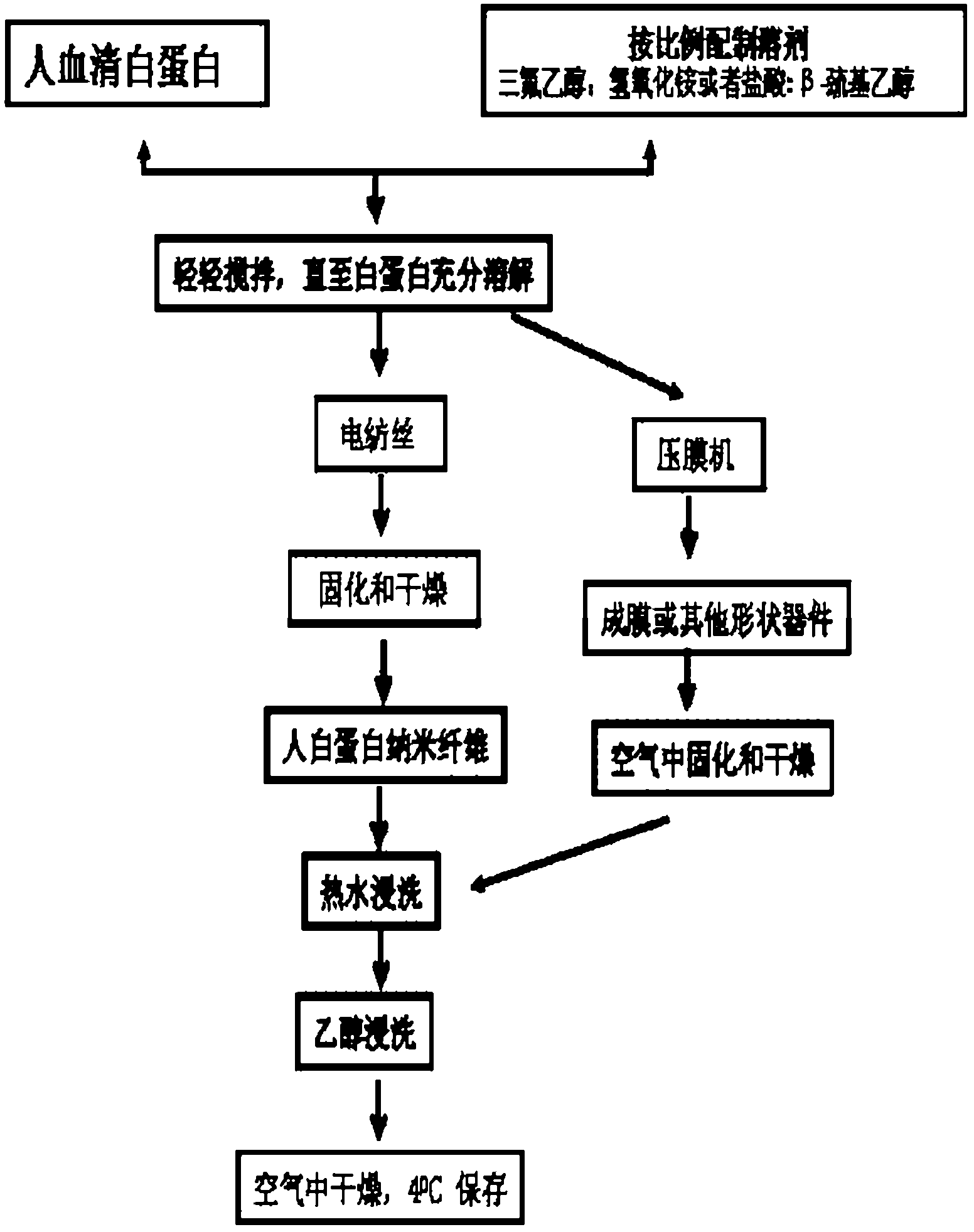
[0045] Prepare a solution system for dissolving albumin: add 0.27ml of 0.1M ammonium hydroxide to 1.74ml of trifluoroethanol, then add 50ul of mercaptoethanol, stir to make it fully mixed. Weigh 0.3 g of recombinant human serum albumin (10%, w / v) and dissolve it in the above solution, stir gently with a magnetic bar for 30 minutes until the albumin is fully dissolved, and pump air with a vacuum pump for 30 minutes to eliminate the bubbles to obtain spinning solution.
[0046] The spinning solution is formed on a collector with a flow rate of 0.3ml per hour through the spinneret hole, an electric field intensity of 1.0KV / cm, a distance between the electrode and the rotating disk of 14cm, and a collector rotating disk edge speed of 8 m / s. After curing and drying for 24 hours in a glass drying dish (humidity 30%), human albumin nanofibers were obtained.
[0047] The albumin fibers were then soaked in deionized hot water at 37°C for 1 hour, then soaked in 35% ethanol prepared fro...
Embodiment 2
[0050]Albumin derived from human plasma (non-genetic recombination) was selected as the matrix material. Prepare a solution system for dissolving albumin: add 0.27ml of 0.1M ammonium hydroxide to 1.74ml of trifluoroethanol, then add 50ul of mercaptoethanol, stir to make it fully mixed. Weigh 0.3 g of recombinant human serum albumin (10%, w / v) and dissolve it in the above solution, stir gently with a magnetic bar for 30 minutes until the albumin is fully dissolved, and pump air with a vacuum pump for 30 minutes to eliminate the bubbles to obtain spinning solution.
[0051] The spinning solution is formed on a collector with a flow rate of 0.3ml per hour through the spinneret hole, an electric field intensity of 1.0KV / cm, a distance between the electrode and the rotating disk of 14cm, and an edge speed of the collector rotating disk of 8 m / s. After curing and drying for 24 hours in a glass drying dish (humidity 30%), human albumin nanofibers were obtained.
[0052] The albumin...
Embodiment 3
[0055] Prepare a solution system for dissolving albumin: add 0.16ml of 0.1M ammonium hydroxide to 1.85ml of trifluoroethanol, then add 50ul of mercaptoethanol, stir to make it fully mixed. Weigh 0.37 g of recombinant human serum albumin (12%, w / v) and dissolve it in the above solution, stir gently with a magnetic bar for 30 minutes until the albumin is fully dissolved, and pump air with a vacuum pump for 30 minutes to eliminate the bubbles to obtain spinning solution.
[0056] The spinning solution is formed on a collector with a flow rate of 0.3ml per hour through the spinneret hole, an electric field intensity of 1.0KV / cm, a distance between the electrode and the rotating disk of 14cm, and an edge speed of the collector rotating disk of 8 m / s. After curing and drying for 24 hours in a glass drying dish (humidity 30%), human albumin nanofibers were obtained.
[0057] The albumin fibers were then soaked in deionized hot water at 37°C for 1 hour, then soaked in 35% ethanol pre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Breaking strength | aaaaa | aaaaa |
| Breaking strength | aaaaa | aaaaa |
| Elongation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com