Synthesis method for 2,3,6,7-triptycene tetracarboxylic dianhydride
A technology of pterylene tetracarboxylic dianhydride and pterylene tetracarboxylic acid, which is applied in the field of functional polymer materials to achieve the effects of prolonging feeding time, high product purity and avoiding side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A kind of synthetic method of 2,3,6,7-triptycenetetracarboxylic dianhydride of the present embodiment comprises the following steps: under conditions of ice-salt bath and vigorous stirring, 35g (grams) of aluminum trichloride is divided into three additions Place 80ml (milliliters) of o-xylene in a 250ml three-necked flask equipped with a mechanical stirrer, slowly add 8g (grams) of benzyl alcohol dropwise through a constant pressure funnel, and keep the system temperature at -5°C during the addition. After the addition is complete, stir at room temperature for 0.5h (hour), and then react in an oil bath at 115°C for 4h. Thereafter, the mixed reactant was settled in 500ml of 5% hydrochloric acid ice water, and after standing still, it was filtered with suction, and the filter cake was washed with 50ml of water, 100ml of ethyl acetate, 50ml of saturated sodium bicarbonate, and 50ml of water, and vacuum-dried to obtain 8.5g of white 2,3,6,7-Tetramethylanthracene is a solid...
Embodiment 2
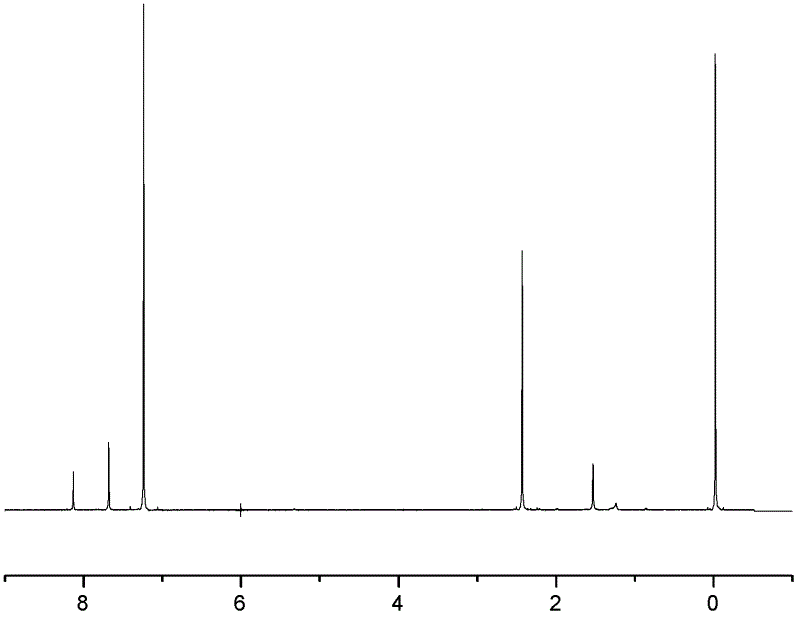
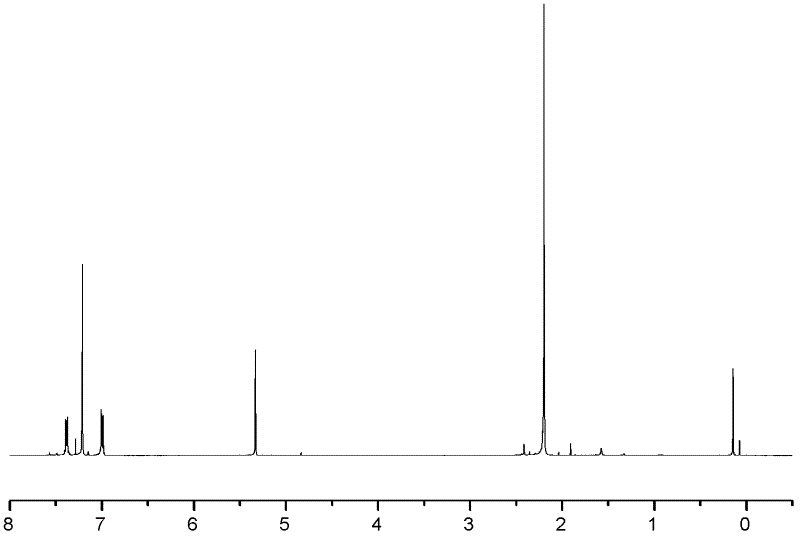
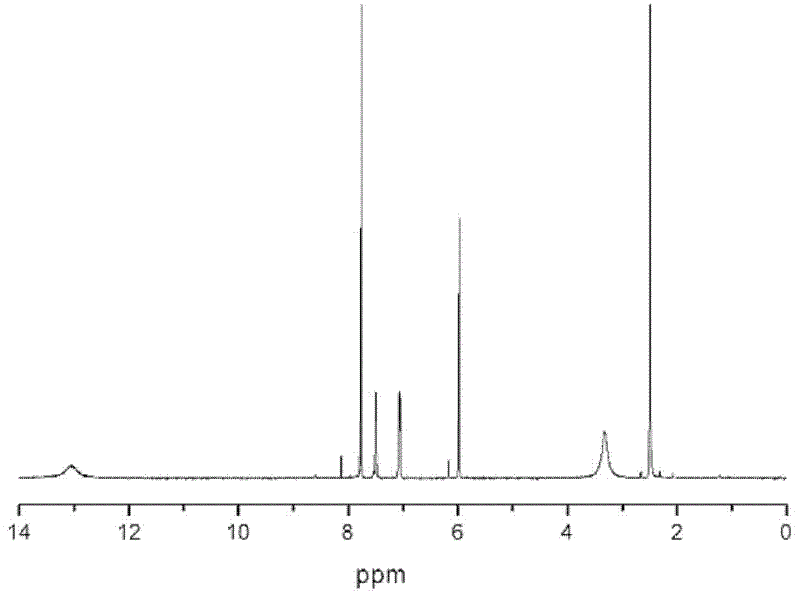
[0046] A kind of synthetic method of 2,3,6,7-triptycene tetracarboxylic dianhydride of the present embodiment comprises the following steps: under ice-salt bath and vigorous stirring condition, put 120ml (milliliters) o-xylene and 70ml (milliliter) in the 250ml three-necked flask of dichloromethane, add 60g (gram) aluminum trichloride in four times, guarantee system temperature 0 ℃ during the addition process. After the addition is complete, react at room temperature for 0.5 h (hour), and then react in a water bath at 65° C. for 4 h. Afterwards, the mixed reactant was slowly dispersed in 300ml of 5% hydrochloric acid ice-water mixed solution. During the pouring process, vigorous stirring was required. After standing still, suction filtration, the filter cake was washed with anhydrous acetone, dried, and o-xylene was recrystallized to obtain white 20 g of flaky 2,3,6,7-tetramethylanthracene solid, melting point is 299°C. figure 1 It is the 1H-NMR chart of 2,3,6,7-tetramethylan...
Embodiment 3
[0060] A kind of synthetic method of 2,3,6,7-triptycene tetracarboxylic dianhydride of the present embodiment comprises the following steps: under conditions of ice-salt bath and vigorous stirring, 40g (grams) of aluminum trichloride is divided into three additions Place 80ml (milliliters) of o-xylene in a 250ml three-necked flask equipped with a mechanical stirrer, slowly add 10g (grams) of benzyl alcohol dropwise through a constant pressure funnel, and keep the system temperature at about -5°C during the addition. After the addition is complete, stir at room temperature for 15 minutes (minutes), and then react in an oil bath at 115° C. for 3.5 hours (hours). Thereafter, the mixed reactant was settled in 65ml of 5% hydrochloric acid ice water, and after standing still, it was suction filtered, and the filter cake was washed with 65ml of water, 125ml of ethyl acetate, 65ml of saturated sodium bicarbonate, and 65ml of water, and vacuum-dried to obtain 9.8g of white 2,3,6,7-Tetr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com