Process for preparing pyrazine-2-formic acid through metalloporphyrin catalytic oxidation of 2-methylpyrazine
A catalytic oxidation, methylpyrazine technology, applied in the direction of organic chemistry, etc., can solve the problems of large toxic waste water, waste liquid, large amount of catalyst, environmental pollution, etc., to reduce energy consumption and operating costs, reduce reaction costs, reduce dangerous effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
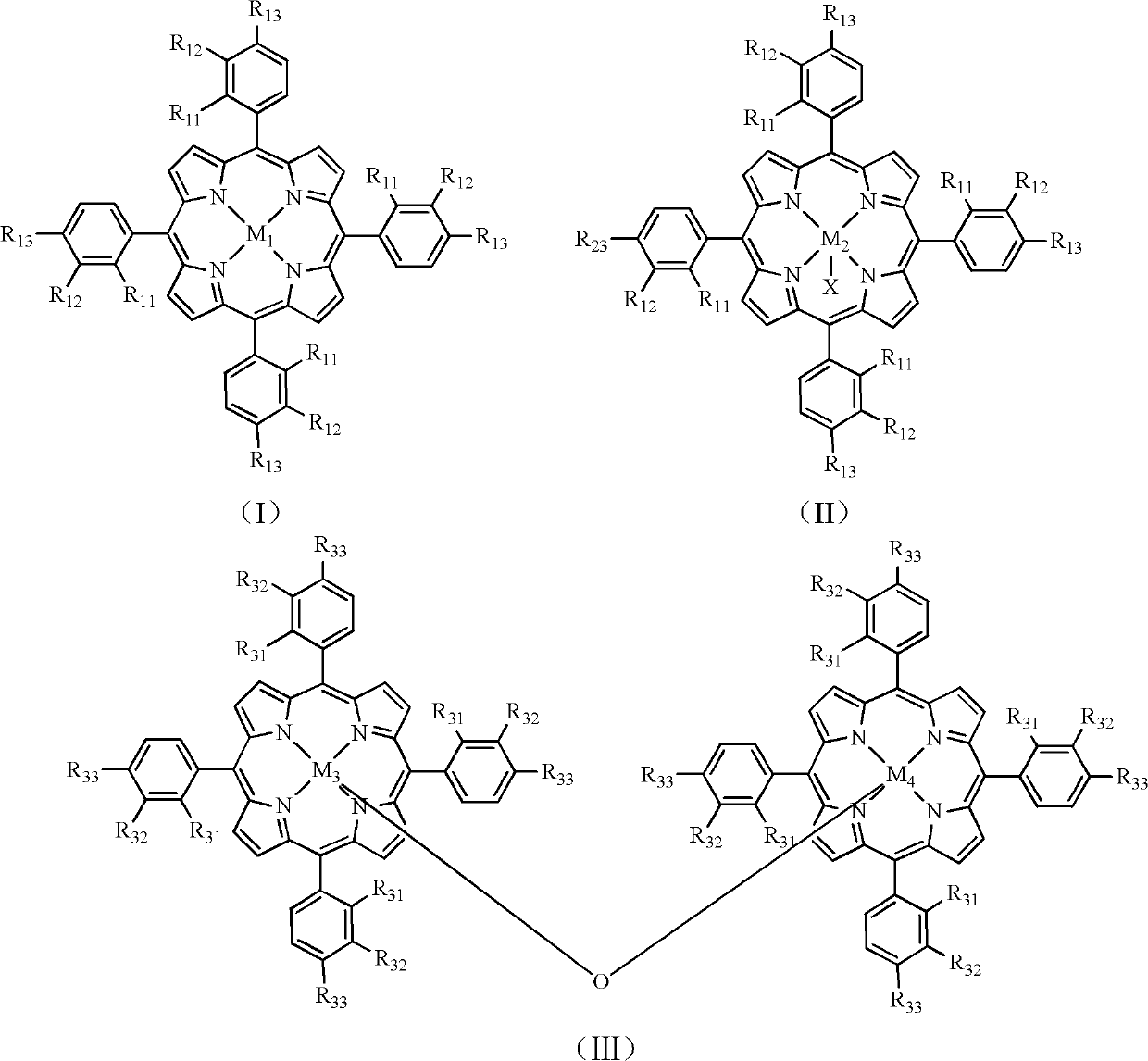
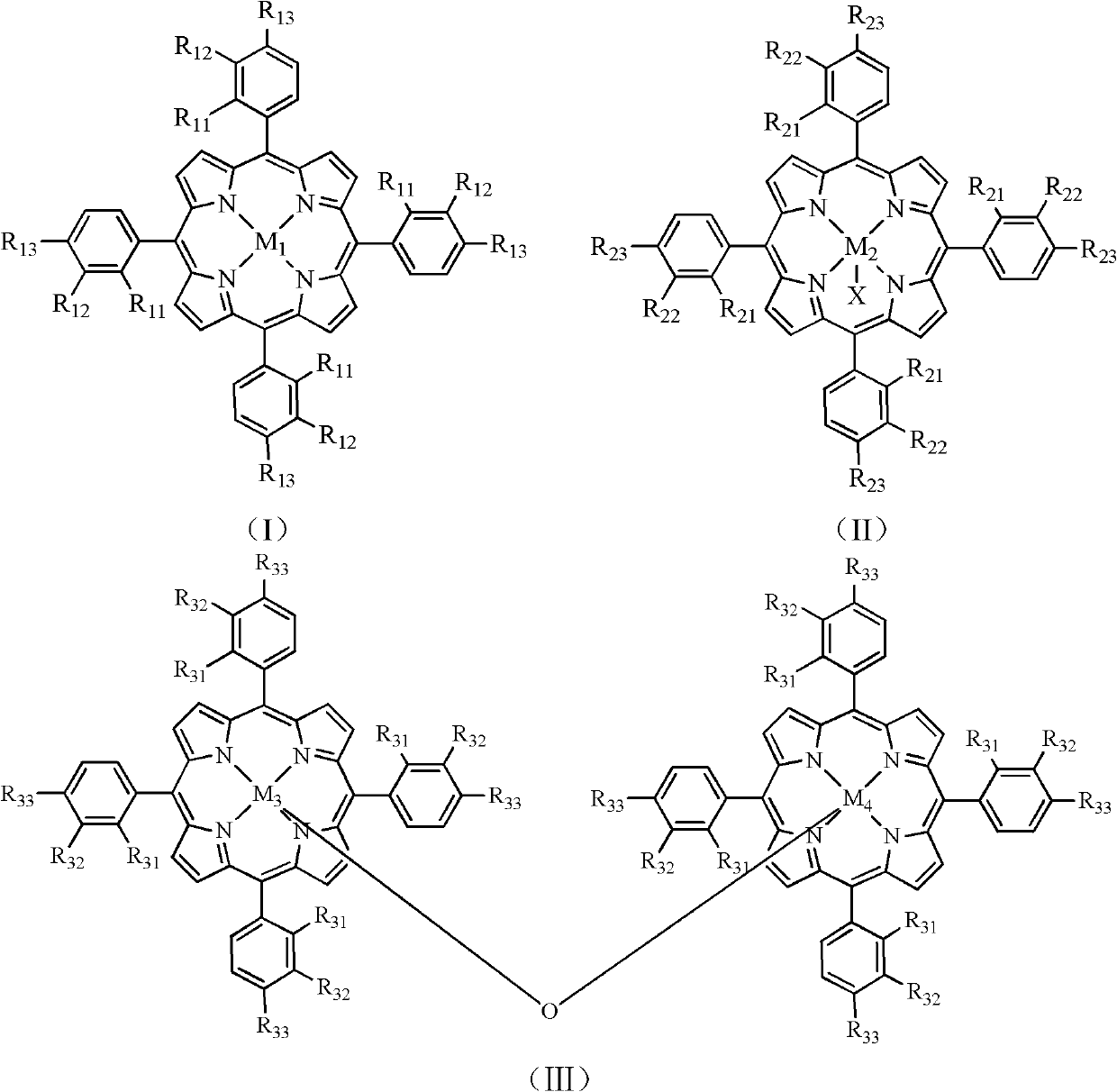
[0021] Add 5.04g potassium hydroxide successively in the autoclave of 200mL, 2.82g 2-methylpyrazine, 30mL ethanol, 0.0014g have the mononuclear metalloporphyrin of formula (I) structure (wherein R 11 =R 12 = H, R 13 = Gl, M 1 =Mn), feed 1.5 MPa of oxygen, and react at 120° C. for 2 hours to obtain pyrazine-2-carboxylic acid. Analysis by high performance liquid chromatography showed that the conversion rate of 2-methylpyrazine was 34.9%, and the yield of pyrazine-2-carboxylic acid was 20.0%.
Embodiment 2
[0023] In the autoclave of 200mL, add 4.20g potassium hydroxide successively, 2.82g 2-methylpyrazine, 30mL ethanol, 0.0014g have the mononuclear metalloporphyrin of formula (II) structure (wherein R 21 =R 22 = H, R 23 = NO 2 , M 2 =Fe, X=Cl), feed 2.0 MPa of oxygen, and react at 120° C. for 0.5 hour to obtain pyrazine-2-carboxylic acid. Analysis by high performance liquid chromatography showed that the conversion rate of 2-methylpyrazine was 14.1%, and the yield of pyrazine-2-carboxylic acid was 9.2%.
Embodiment 3
[0025] In the autoclave of 200mL, add 3.36g potassium hydroxide successively, 2.82g 2-methylpyrazine, 29.7mL ethanol, 0.3mL water, 0.0010g have the mononuclear metalloporphyrin of formula (I) structure (wherein R 11 =R 12 = H, R 13 = COOH, M 1 =Co), feed 2.5MPa of oxygen, and react at 100° C. for 1 hour to obtain pyrazine-2-carboxylic acid. Analysis by high performance liquid chromatography showed that the conversion rate of 2-methylpyrazine was 16.3%, and the yield of pyrazine-2-carboxylic acid was 10.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com