Method for synchronously synthesizing hydrogen peroxide and peroxyacetic acid
A technology of peracetic acid and hydrogen peroxide, applied in the direction of peroxide/peroxyhydrate/peroxyacid/superoxide/ozonide, preparation of peroxygen compounds, chemical instruments and methods, etc., can solve the problem of corrosion Equipment corrosion resistance requirements and other issues, to achieve the effect of small corrosion, low content, simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
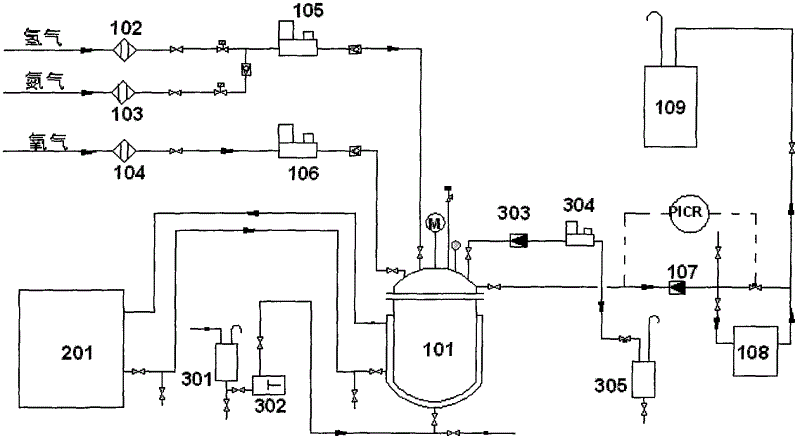
[0031] In the mixed solvent that synthetic reaction adopts, the mass ratio of ethanol, acetic acid and water is 60: 12: 28, and phosphoric acid content is 0.0050%, adds sodium bromide to make Br in the system - The content is 0.0100%, and the above mixed solvent and palladium catalyst (the mass ratio of mixed solvent and catalyst is 80:1) are added into the reactor, and the mass fraction of palladium in the catalyst is 5%. The reaction temperature is controlled at -20° C., the reaction pressure is controlled at 1 MPa, and the intake ratio of hydrogen and oxygen is 5:95 (volume ratio under standard conditions). Continuously react for 128h on the device shown in the accompanying drawing. After testing, the mass fraction of hydrogen peroxide in the mixed solution was 15.2%, and the mass fraction of peracetic acid was 8.2%.
Embodiment 2
[0033] In the mixed solvent that synthetic reaction adopts, the mass ratio of ethanol, acetic acid and water is 95: 0.5: 4.5, and phosphoric acid mass fraction is 0.0900%, adds hydrogen bromide to make Br - The mass fraction is 0.0005%, and the above-mentioned mixed solvent and palladium catalyst (the mass ratio of the mixed solvent and the catalyst is 100:1) are added into the reactor, and the mass fraction of palladium in the catalyst is 2.5%. The reaction temperature is controlled at 10° C., the reaction pressure is controlled at 20 MPa, and the intake ratio of hydrogen and oxygen is 40:60 (volume ratio under standard conditions). in the attached figure 1 The continuous reaction on the device shown was 128h. After testing, the content of hydrogen peroxide in the mixed liquid product is 14.1%, and the content of peracetic acid is 3.1%.
Embodiment 3
[0035] In the mixed solvent used in the synthesis reaction, the mass ratio of ethanol, acetic acid and water is 80:8:12, the mass fraction of phosphoric acid is 0.0600%, the Br -The mass fraction is 0.0050%, and the above mixed solvent and palladium catalyst (the mass ratio of the mixed solvent to the catalyst is 80:1) are added into the reactor, and the mass fraction of palladium in the catalyst is 5%. The reaction temperature is controlled at -10° C., the reaction pressure is controlled at 10 MPa, and the intake ratio of hydrogen and oxygen is 20:80 (volume ratio under standard conditions). in the attached figure 1 The continuous reaction on the device shown was 224h. After testing, the mass fraction of hydrogen peroxide in the mixed solution was 15.4%, and the mass fraction of peracetic acid was 7.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com