CdTe quantum dot-containing nano-artesunate capsule and its preparation method
A technology of artesunate and quantum dots, which is applied in the direction of medical preparations containing active ingredients, capsule delivery, microcapsules, etc., can solve the problems of fast metabolism, inconvenient clinical application, and low bioavailability, and achieve simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
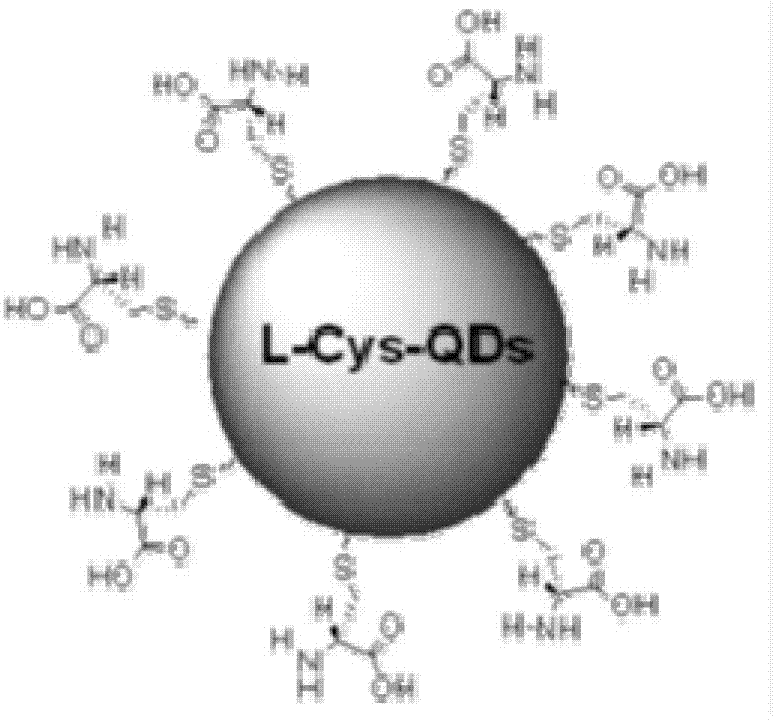
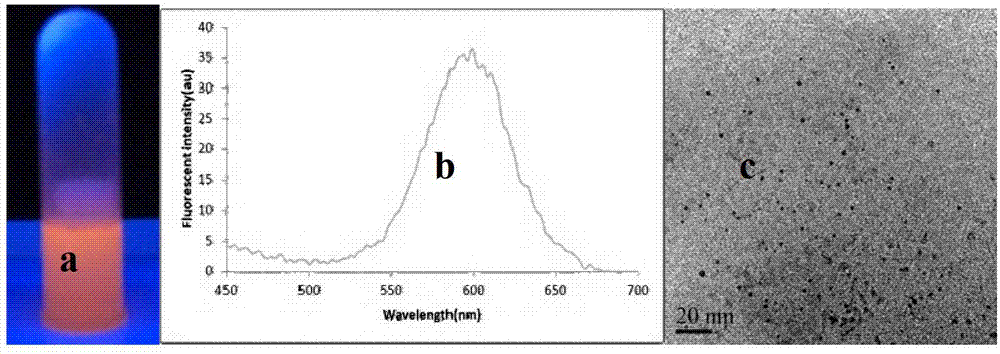
[0025] Quantum dots are semiconductor crystals with a nanometer size. Due to the very small particle size, most of the atoms are located on the surface of the quantum dots. The increase in the number of surface atoms leads to insufficient coordination of surface atoms, and an increase in unsaturated bonds and dangling bonds. The surface atoms are highly active, extremely unstable, and easily combined with other atoms. In order to improve its stability and increase compatibility with artesunate, the applicant added L-cysteine when synthesizing CdTe quantum dots to obtain cysteine-adsorbed CdTe quantum dots (see figure 1 ), with a particle size between 2.5 and 5.0nm, water-soluble CdTe quantum dots with strong fluorescence (such as figure 2 ).
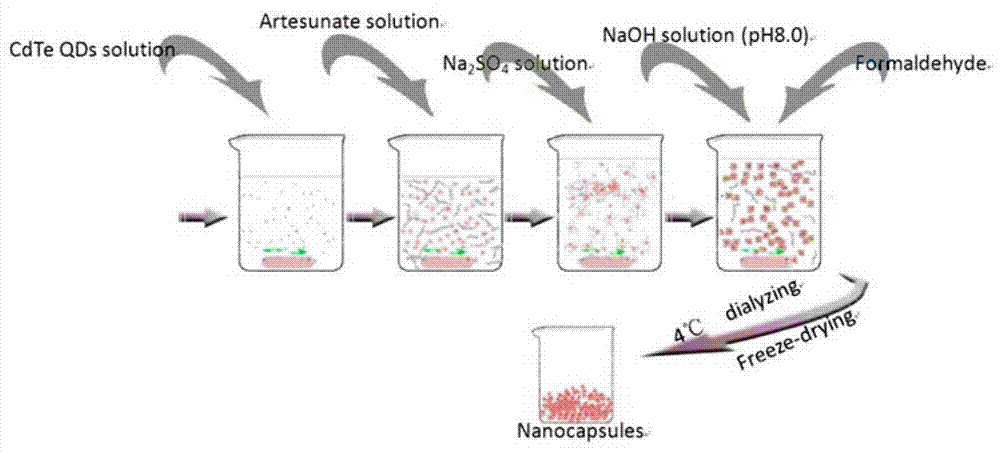
[0026] The invention adopts the nascent microcrystal method, utilizes the self-organization method to realize three-layer nano packing, and prepares the nano artesunate capsule containing quantum dots. refer to image 3 , the speci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com