Method for preparing sterile cefmenoxime hydrochloride compound
A technology for cefmenoxime hydrochloride and a compound, which is applied in the field of medicine, can solve the problems of low purity of cefmenoxime hydrochloride, does not meet medical requirements, low product yield, etc., and achieves shortened crystal growth time, saved production cycle, and high purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
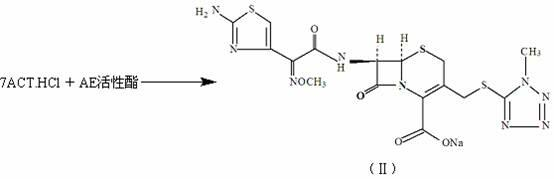
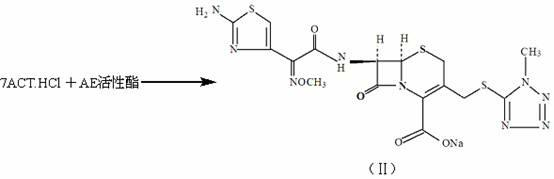
[0033] Embodiment 1: the synthesis of cefmenoxime sodium salt
[0034] Add 364.5L of dichloromethane, 36.45kg of 7-ACT·HCl, and 42.00kg of AE active ester into a 1000L glass-lined reaction tank in sequence. Under stirring, add an equal amount of alkalizing agent to react at room temperature for 3 hours, add water 4×100L for extraction, and combine water Phase, decolorize 20 minutes with activated carbon 2kg and aluminum oxide 16kg, generate 7-[α-(2-aminothiazol-4-yl)-Z-2-methoxyiminoacetamido]-3-(1-methyl -1H-5-tetrazolyl-thiomethyl)-3-cephem-4-carboxylic acid sodium salt solution (ie cefmenoxime sodium salt) (compound II).
Embodiment 2
[0035] Embodiment 2: the preparation of cefmenoxime hydrochloride
[0036] Aseptically filter the decolorized aqueous solution into a 1000L glass-lined crystallization tank, wash the charcoal layer with water, combine the filtrates, adjust the pH to 2.0 with 1.0mol / L hydrochloric acid, grow the crystals for 1 hour, wash the filtered crystals with water, and vacuum at 40°C After drying, 47.39kg of cefmenoxime hydrochloride was obtained, with a yield of 130% and a purity of 99.60% by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com